Transdermal Delivery & Localized Blood Flow Research: Innovations, Mechanisms, and Clinical Implications
Exploring how skin physiology and emerging methods converge to enhance drug uptake.
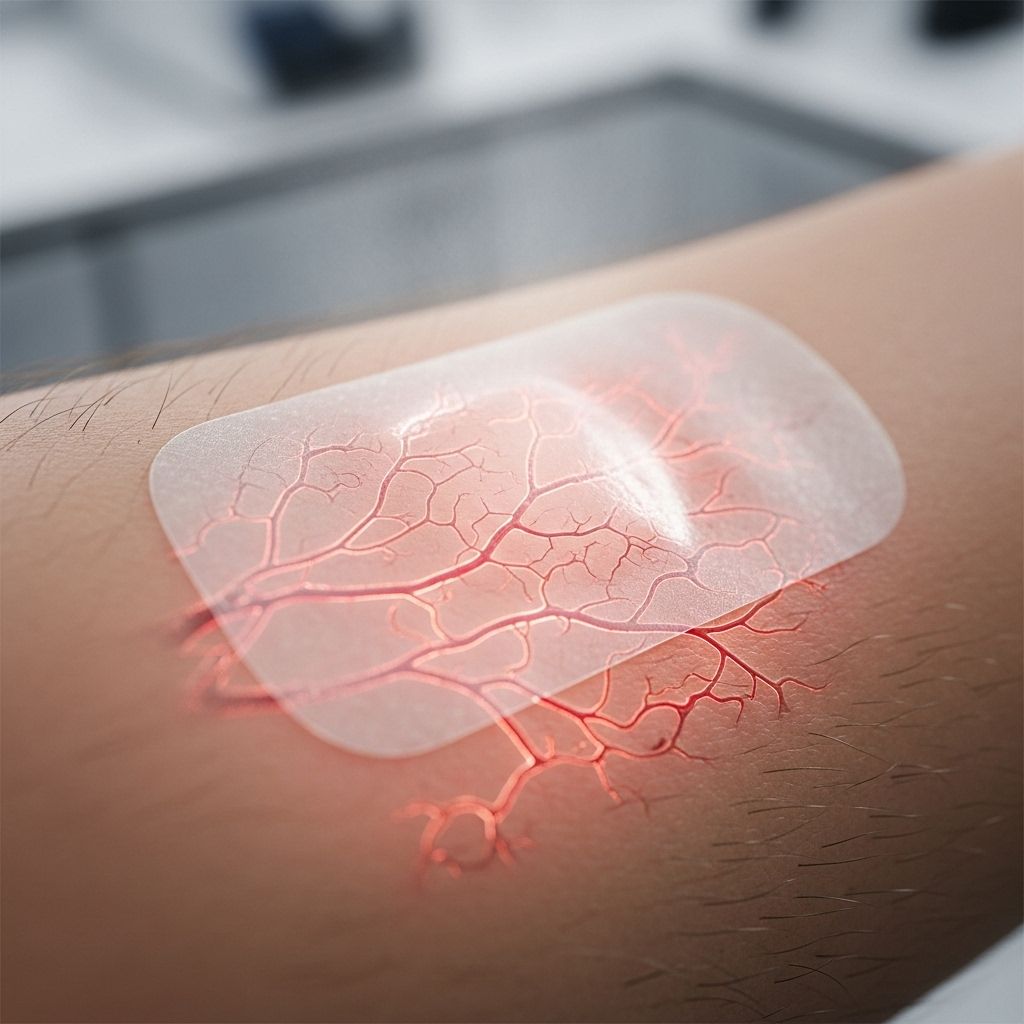
Transdermal drug delivery systems (TDDS) have advanced significantly in recent decades, revolutionizing the way medications are administered. By leveraging the skin as a portal, these systems provide a non-invasive, controlled, and often safer alternative to traditional drug administration. One of the most critical variables influencing transdermal efficacy is localized blood flow—a factor with significant physiological and clinical implications. This comprehensive article explores the intricate relationship between transdermal delivery and blood flow, current innovations, and the evolving landscape of transdermal therapeutics.
Table of Contents
- Introduction to Transdermal Delivery
- Skin Structure and the Barrier to Transdermal Delivery
- Mechanisms of Transdermal Drug Transport
- Role of Localized Blood Flow in Transdermal Delivery
- Enhancement Techniques for Transdermal Transport
- Current Transdermal Delivery Systems and Innovations
- Clinical and Therapeutic Benefits
- Challenges and Limitations
- Future Directions in Research
- Frequently Asked Questions (FAQs)
Introduction to Transdermal Delivery
Transdermal drug delivery (TDD) refers to the process of delivering medications through the skin for systemic or localized therapeutic effects. Unlike oral or intravenous routes, transdermal systems bypass the digestive tract and hepatic first-pass metabolism, facilitating steady plasma drug levels, minimizing side effects, and improving patient compliance.
Examples include nicotine patches for smoking cessation, hormone therapies, pain management drugs, and cardiovascular medications.
Skin Structure and the Barrier to Transdermal Delivery
The skin is a multilayered organ composed of:
- Epidermis: The outermost layer; includes the stratum corneum (a tough, keratin-rich barrier).
- Dermis: Middle, vascularized layer containing hair follicles, sweat, and sebaceous glands.
- Hypodermis (subcutaneous tissue): The innermost, fatty connective tissue layer.
The stratum corneum acts as the primary barrier, with 10–20 layers of dead, densely packed cells and lipid matrices that restrict the movement of drugs into deeper skin tissues.
Routes of Transdermal Drug Penetration
- Transcellular (through cells): Drug molecules pass directly through corneocytes.
- Intercellular (between cells): Molecules diffuse around potential gaps between keratinocytes.
- Transappendageal: Via skin appendages (hair follicles, sweat ducts).
Despite all three routes, the limited surface area and constant turnover of skin cells make effective penetration a major challenge.
Mechanisms of Transdermal Drug Transport
Successful transdermal delivery is generally governed by:
- Passive Diffusion: Fick’s Law describes the drug diffusion based on concentration gradients, skin thickness, and permeability.
- Skin Permeability: Highly influenced by the physicochemical properties of the drug (size, charge, lipophilicity).
- Local Blood Flow: Drives the absorption of drugs into capillary plexuses and systemic circulation once drugs cross the skin’s barrier.
In other words, the rate and extent of transdermal drug absorption are dictated primarily by skin permeability and the efficacy of blood flow beneath the skin to clear absorbed drugs into the circulation.
Role of Localized Blood Flow in Transdermal Delivery
Localized blood flow refers to the movement of blood through dermal and subdermal vessels directly under the application site. This process is crucial for several reasons:
- Drug Uptake Control: Increased blood flow enhances drug uptake from the dermal layer and minimizes local drug accumulation.
- Rate-Limiting Step: In drugs with rapid skin permeation, dermal blood flow can be the rate-limiting step for systemic absorption.
- Thermal Modulation: Heat application induces local vasodilation, sometimes increasing blood flow many-fold, which in turn accelerates drug absorption and systemic bioavailability.
Key Research Findings:
- Local heating of the skin increases plasma concentrations of transdermally delivered drugs, likely via increased skin permeability and blood vessel dilation.
- Thermal, ultrasound, and electrical stimulation can further modulate localized blood flow and drug delivery rates.
Summary Table: Effects of Localized Blood Flow on Transdermal Drug Delivery
| Method | Effect on Blood Flow | Result on Drug Delivery |
|---|---|---|
| Local Heat | Vasodilation; increased perfusion | Higher drug absorption; enhanced plasma drug levels |
| Ultrasound | Temporary vessel dilation | Greater diffusion and systemic uptake |
| Iontophoresis | Minimal change | Electrophoretic drug transport, increased by current intensity |
| Occlusion (patch cover) | Induces mild local warming and humidity | Slightly increased permeation |
Enhancement Techniques for Transdermal Transport
Technological advances have overcome key barriers in traditional transdermal systems by enhancing skin permeability, modifying local physiological environments, and utilizing external energy sources. Below are the main approaches:
- Microneedles: Tiny projections painless pierce the stratum corneum, delivering higher-molecular-weight (large) drugs with minimal discomfort.
- Iontophoresis: Uses a low electric current to drive charged drugs through the skin, allowing programmable delivery rates.
- Electroporation: Short high-voltage pulses create micropores in the epidermis, facilitating passage of large molecules.
- Thermal Poration: Heat (via warming patches or devices) increases barrier permeability and local capillary flow, dramatically raising drug delivery rates.
- Chemical Enhancers: Solvents or surfactants temporarily disrupt the lipid matrix of the stratum corneum, increasing diffusion.
- Ultrasound (Phonophoresis): Sound waves facilitate drug penetration and increase skin blood flow, further improving absorption.
Current Transdermal Delivery Systems and Innovations
Contemporary TDDS are available in multiple forms, each optimized for specific drugs and target outcomes:
- Patches: Reservoir or matrix systems for steady, prolonged release.
- Gels and Creams: Spreadable formulations for localized or systemic therapy.
- Sprays and Liquid Bandages: Rapid, metered dosing and sustained release from a superficial reservoir.
- Micro-reservoirs: Advanced miniaturized systems targeting controlled release.
- Smart Patches: Integrate sensors and electronics for adjustable, responsive dosing (e.g., glucose-sensing patches for insulin).
Examples include contraceptive, analgesic, anti-hypertensive, and hormone replacement therapy patches.
Comparison Table: Traditional vs Smart Transdermal Systems
| Type | Key Feature | Delivery Control | Blood Flow Impact |
|---|---|---|---|
| Traditional Patch | Passive diffusion | Fixed, non-adjustable | Dependent on skin perfusion |
| Smart Patch | Sensors/Microprocessors | Programmable, on-demand | May include feedback on flow |
| Iontophoretic Device | Current-driven | User or device modulated | Independent of blood flow rate |
Clinical and Therapeutic Benefits
- Non-invasive Delivery: Reduced infection risk and needle phobia compared to injections.
- Bypasses First-Pass Metabolism: Direct entry to systemic circulation, enhancing the bioavailability of many drugs.
- Improved Patient Compliance: Fewer doses needed, convenient usage, rapid discontinuation if required.
- Steady Drug Levels: Avoids peaks and troughs associated with oral or injectable regimens.
- Enables Therapies Infeasible by Other Routes: Supports long-acting medications and drugs with narrow therapeutic windows.
Challenges and Limitations
Despite advances, several key obstacles remain:
- Skin Barrier: Limits delivery of high molecular weight or hydrophilic drugs.
- Inter-Individual Variation: Differences in skin thickness, hydration, age, disease, and local blood flow affect absorption rates.
- Local Irritation: Extended exposure to adhesive patches or chemical enhancers can cause dermatitis or allergic reactions.
- Poor Suitability for Immediate Effect: Slower onset compared to intravenous administration in emergencies.
Future Directions in Research
Active research areas include:
- Personalized TDDS: Devices and patches that dynamically adjust delivery based on real-time sensor data (skin temperature, blood flow, biomarkers).
- Molecular Engineering: Development of new chemical enhancers and nanoparticle carriers to improve skin penetration.
- Integration with Digital Health: Remote monitoring and control of transdermal devices via smartphones and wearable technologies.
- Theranostic Patches: Dual-function patches capable of diagnosis and therapy (e.g., glucose sensors with insulin delivery).
- Tissue-Targeted Delivery: Patches that direct medication to specific local regions by exploiting variations in local blood flow.
Ongoing translational research will continue to refine strategies for optimizing local blood flow and transdermal system efficacy, broadening the scope of treatable diseases and improving patient outcomes.
Frequently Asked Questions (FAQs)
Q: What are the main advantages of transdermal drug delivery?
A: Key advantages include non-invasiveness, avoidance of first-pass metabolism, steady plasma drug levels, minimized systemic side effects, and enhanced patient compliance.
Q: How does heat improve transdermal drug delivery?
A: Heat increases local blood flow and skin permeability, accelerating drug diffusion into the systemic circulation and resulting in higher plasma concentrations of transdermally-delivered medications.
Q: Are all drugs suitable for transdermal delivery?
A: No. Ideal candidates have low molecular weight, favorable lipophilicity, low required dosage, and high potency. Hydrophilic or high-molecular-weight drugs often require enhancement techniques to be transdermally viable.
Q: Can blood flow at the application site be increased intentionally?
A: Yes. Application of local heat, ultrasound, and some chemical enhancers can vasodilate dermal blood vessels, significantly raising local perfusion and accelerating drug uptake.
Q: Is transdermal delivery the best option for rapid drug action?
A: Not always. While beneficial for sustained and consistent drug levels, the rate of absorption may not match that of intravenous or some oral therapies, especially for urgent treatment needs.
References
1. Hull, Wade. “Heat-Enhanced Transdermal Drug Delivery: A Survey Paper.” JARCET.
2. StatPearls. “Transdermal Medications – Mechanism of Action.” NCBI Bookshelf.
3. Prausnitz, M.R. et al. “Transdermal drug delivery – PMC.”
4. Wiley Online Library. “Innovative Transdermal Drug Delivery Systems.”
References
- https://www.jarcet.com/articles/Vol2Iss1/Hull.htm
- https://www.ncbi.nlm.nih.gov/books/NBK556035/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC2700785/
- https://onlinelibrary.wiley.com/doi/full/10.1002/bmm2.70001
- https://www.frontiersin.org/journals/medical-technology/articles/10.3389/fmedt.2025.1552294/full
Read full bio of medha deb












