Trace Minerals and the Assembly of Tight Junctions: Focus on the Critical Role of Zinc in Barrier Integrity and Function
Coordinating mineral-driven protein assembly to maintain cell cohesion and tissue health.
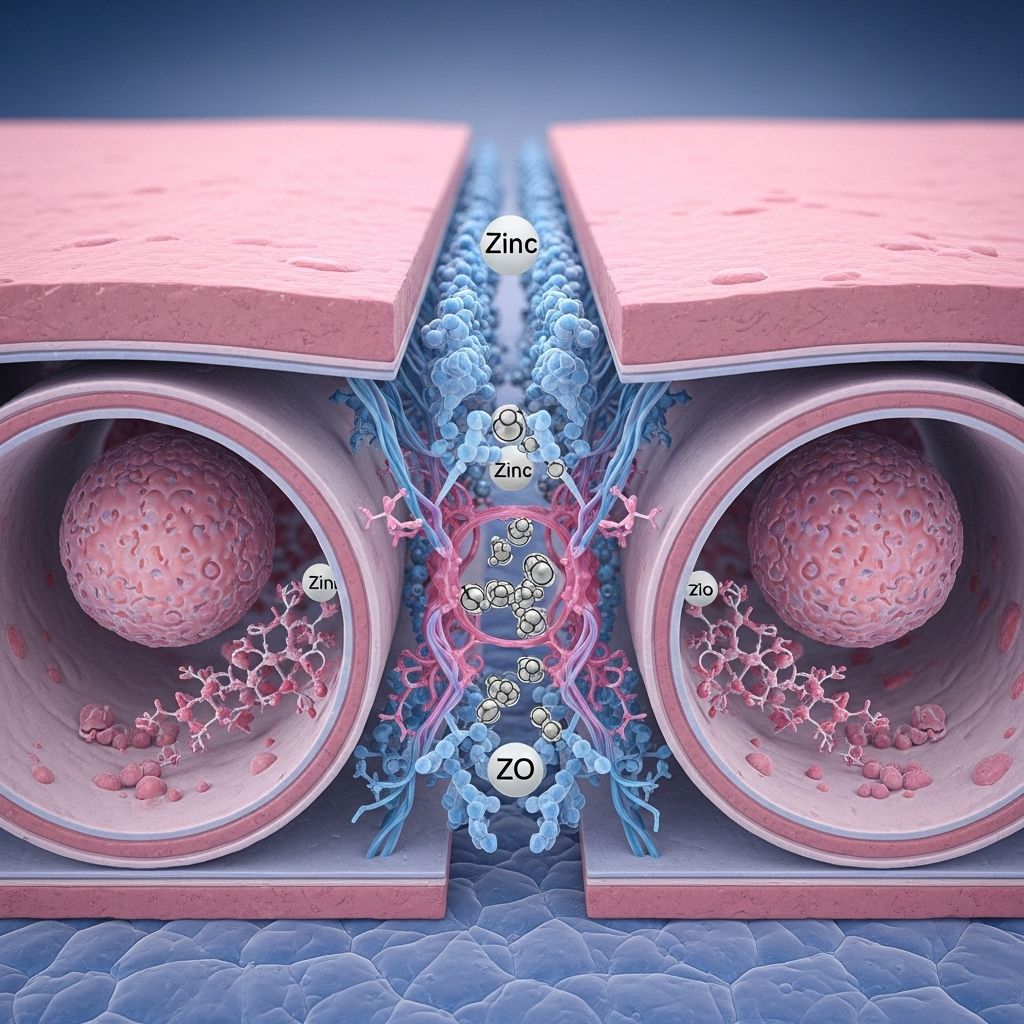
Trace Minerals and the Assembly of Tight Junctions: The Essential Role of Zinc
Trace minerals play exceptional roles in cellular physiology, with a growing body of evidence highlighting their influence on cell–cell adhesion, signaling, and barrier function. Among these, zinc has emerged as a pivotal regulator in the organization and maintenance of tight junctions (TJs), essential structures that govern paracellular permeability and tissue homeostasis.
Table of Contents
- Introduction to Tight Junctions
- What Are Trace Minerals?
- Tight Junction Structure and Function
- Molecular Components of Tight Junctions
- Role of Trace Minerals in Tight Junction Assembly
- Zinc: A Key Player in Tight Junction Integrity
- Zinc and Calcium: Comparative Insights in TJ Modulation
- Zinc Deficiency and Barrier Disruption
- Therapeutic Potential of Zinc in Barrier-Related Disorders
- Future Directions for Research
- Frequently Asked Questions
Introduction to Tight Junctions
Tight junctions (TJs) are specialized intercellular structures that govern the permeability of epithelial and endothelial cell layers. Their primary function is to create a selective barrier, regulating the movement of ions, water, and solutes across tissues and thus maintaining compartmentalization within the body .
What Are Trace Minerals?
Trace minerals, also known as trace elements, are minerals required by organisms in minute amounts for critical biological processes. Though required in low concentrations, they profoundly influence cellular machinery, acting as cofactors for enzymes, modulating structural features, and impacting signaling cascades.
- Zinc (Zn): Essential for protein structure, enzymatic catalysis, and cell signaling.
- Copper (Cu): Participates in oxidative stress mitigation and redox reactions.
- Magnesium (Mg): Required for ATP stability and functional protein assemblies.
- Selenium (Se), Iron (Fe), and others: Critical for antioxidant defenses and cellular metabolism.
Tight Junction Structure and Function
Tight junctions form a continuous, mesh-like network around the apex of epithelial cells. Their primary roles include:
- Barrier Function: Prevent paracellular diffusion of water and solutes, maintaining selective permeability and tissue compartmentalization .
- Fence Function: Restrict the movement of membrane proteins and lipids, preserving cell polarity and specialized domains.
- Signaling Platform: Serve as hubs for signaling cascades that regulate cell proliferation, differentiation, and immune responses.
Molecular Components of Tight Junctions
Tight junctions comprise a multiprotein complex with both transmembrane and cytoplasmic proteins, all interacting with the underlying cytoskeleton .
- Core Transmembrane Proteins:
- Claudins: Major structural and functional elements (at least 27 isoforms in mammals), determine ion selectivity and permeability.
- Occludin: Modulates junction stability and barrier properties.
- Junctional Adhesion Molecules (JAMs): Mediate cell-cell adhesion and signaling.
Intermittent fasting may have significant benefits on intestinal barrier health and tight junction integrity. Learn about the underlying mechanisms and potential advantages by exploring our research on intermittent fasting and intestinal tight junctions.- Cytoplasmic Scaffolding Proteins:
- Zonula Occludens (ZO-1, ZO-2, ZO-3): Anchor TJs to the actin cytoskeleton; critical for assembly and maintenance.
The interplay of these proteins supports dynamic TJ assembly, with precise regulation dependent on environmental cues, mechanical stress, and nutrient status .
Barrier Properties and Permeability
TJs can either form ‘tight’ or ‘leaky’ barriers, depending on protein composition:
| Barrier Type | Properties | Examples |
|---|---|---|
| Tight (high-resistance) | Blocks most solutes and ions; high selectivity | Blood-brain barrier, renal collecting duct |
| Leaky (low-resistance) | Permits controlled diffusion of ions and small molecules | Proximal renal tubule, intestinal epithelium |
Role of Trace Minerals in Tight Junction Assembly
Beyond serving as cofactors for enzymes, trace minerals shape TJ assembly and function via:
- Regulating the expression of TJ proteins (e.g., claudins, occludin)
- Modulating the trafficking and phosphorylation of TJ components
- Supporting cytoskeletal rearrangement and junctional remodeling
Overview of Trace Mineral Influences
- Magnesium: Critical for renal tight junction complexes (claudin-16 and claudin-19) and paracellular magnesium reabsorption .
- Copper: Absorbed transcellularly; influences cellular redox status and can modulate junctional protein oxidation .
- Calcium: Central to cadherin function and contributes to the interplay between tight and adherens junctions .
- Zinc: Detailed below as a master regulator of TJ assembly and function.
Zinc: A Key Player in Tight Junction Integrity
Zinc is a divalent cation involved in over 300 enzymatic reactions and is crucial for gene transcription, protein folding, cell division, and immune signaling. Its pivotal role in tight junction dynamics is now well established through several molecular mechanisms:
1. Regulation of Tight Junction Protein Expression
- Zinc modulates the transcription of genes encoding key TJ proteins such as claudins, occludin, and ZO-1.
- It acts as an essential cofactor for transcription factors (e.g., zinc-finger proteins) that govern TJ-associated gene networks.
2. Post-Translational Stabilization and Assembly
- Zinc facilitates proper folding and conformational stability of TJ proteins, enhancing their incorporation into the tight junction complex.
- It supports protein-protein interactions necessary for the assembly and maintenance of the paracellular barrier.
3. Modulation of Intracellular Signaling
- Zinc serves as a second messenger in signaling pathways that regulate cytoskeletal rearrangements, crucial for the dynamic remodeling of TJs under mechanical and metabolic stress.
- It interacts with kinases and phosphatases that modulate TJ protein phosphorylation and trafficking.
4. Antioxidant and Anti-inflammatory Protection
- Zinc protects TJ proteins from oxidative and inflammatory injury, preserving barrier integrity during physiological and pathological challenges.
Zinc and Calcium: Comparative Insights in TJ Modulation
| Mineral | Main TJ-Related Effects | Key Mechanisms |
|---|---|---|
| Zinc | Upregulates TJ protein expression, promotes assembly, stabilizes proteins, and acts as an antioxidant | Cofactor for transcription factors, enzymatic systems, and second-messenger pathways |
| Calcium | Mediates cell-cell adhesion (via cadherins) and modulates interplay between tight and adherens junctions | Initiates conformational changes in adhesion molecules; triggers signaling cascades influencing TJ reorganization |
Both minerals are crucial, but zinc uniquely orchestrates the synthesis, assembly, and long-term stability of TJ complexes, especially under conditions of oxidative or inflammatory stress .
Zinc Deficiency and Barrier Disruption
Deficiency of zinc impairs epithelial and endothelial barriers through:
- Downregulation of claudins, occludin, and ZO proteins
- Increased paracellular leakiness and loss of tissue polarity
- Heightened susceptibility to inflammation, infection, and tissue injury
Consequences are most apparent in the gut, lungs, kidneys, and blood-brain barrier—systems highly reliant on precise paracellular regulation.
Mechanisms of TJ Disruption in Zinc Deficiency
- Reduced synthesis and assembly of TJ proteins due to impaired gene expression
- Loss of cytoskeletal anchoring, leading to unstable or fragmented junctions
- Inadequate defense against oxidative modification and proteolytic degradation
Therapeutic Potential of Zinc in Barrier-Related Disorders
Restoration of adequate zinc levels is a promising therapeutic approach in conditions marked by impaired tight junction function, such as:
- Inflammatory Bowel Disease (IBD): Zinc supplementation restores barrier integrity and reduces inflammation.
- Infectious Diarrhea: Zinc improves recovery of the intestinal barrier, lowering morbidity and mortality in children.
- Respiratory and Renal Disorders: Beneficial effects on pulmonary and renal epithelia by stabilizing TJs.
These findings have widespread implications for personalized nutrition, public health, and the management of chronic diseases with a barrier dysfunction component.
Future Directions for Research
- Clarifying the precise molecular interactions between zinc, transcriptional regulators, and TJ proteins
- Uncovering potential synergies or antagonisms among trace minerals in barrier regulation
- Developing targeted mineral supplementation strategies based on individual genetic and metabolic profiles
- Investigating the interplay of zinc and immune modulators in chronic and acute barrier-disrupting diseases
Frequently Asked Questions (FAQs)
What are tight junctions and why are they important?
Tight junctions are complexes of proteins at the interface of epithelial and endothelial cells that seal the gap between cells, preserving tissue compartmentalization and controlling the passage of molecules between body compartments. They are vital for organ function and defense against pathogens .
How does zinc affect tight junctions?
Zinc enhances the synthesis, assembly, and stabilization of tight junction proteins, positively influencing the integrity and function of cellular barriers. Its absence leads to disrupted junctions and impaired defenses against stress and infection.
Can zinc supplementation improve diseases linked to barrier dysfunction?
Yes. Zinc supplementation is beneficial in several conditions, including inflammatory bowel disease and infectious diarrhea, helping restore tight junction integrity and reduce disease severity.
Are other trace minerals also important for tight junctions?
Yes. Magnesium, copper, calcium, selenium, and other trace elements all contribute to tight junction composition and function, but their specific roles and mechanisms vary depending on tissue context and physiological need.
What are the signs of zinc deficiency-related barrier breakdown?
Common signs include increased risk of diarrheal illness, impaired wound healing, higher susceptibility to infections, and in some cases, neurological symptoms due to blood-brain barrier compromise.
References
- https://www.gwfnutrition.com/pages/knowledge-base-mineral-trace-element-absorption-mechanisms
- https://pmc.ncbi.nlm.nih.gov/articles/PMC11128289/
- https://rupress.org/jcb/article/221/4/e202105107/213049/Mechanosensitive-calcium-flashes-promote-sustained
- https://clinical-laboratory-diagnostics.com/k10.html
- https://pmc.ncbi.nlm.nih.gov/articles/PMC3337967/
- https://febs.onlinelibrary.wiley.com/doi/10.1002/1873-3468.14252
Read full bio of Sneha Tete












