Tight Junctions vs Adherens Junctions: Comparative Roles in the Gut Barrier and Intestinal Health
Cells lining the intestine unite to control permeability and reinforce digestive defenses.
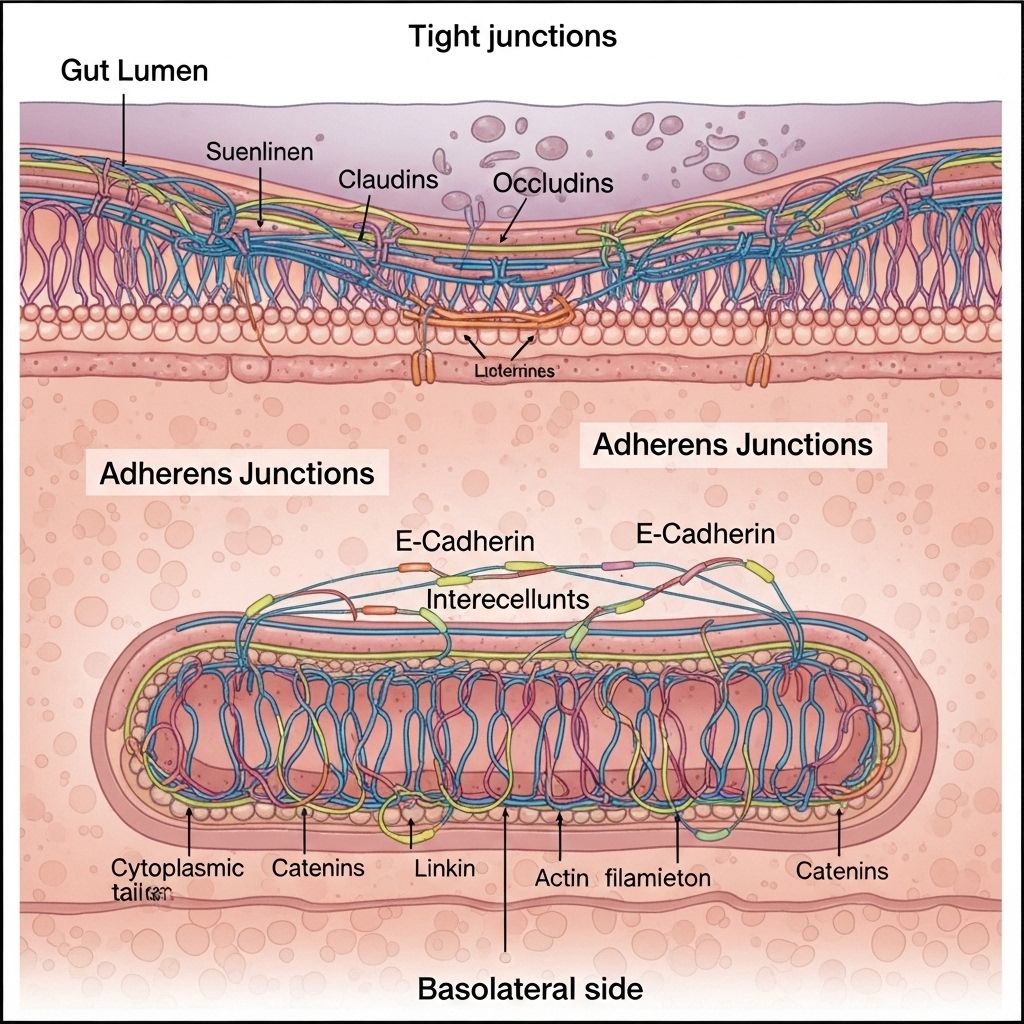
The human gut is lined with a complex epithelial barrier that guards internal physiology against a vast array of external threats, while also facilitating the selective absorption of nutrients and water. Central to this barrier function are specialized structures known as tight junctions and adherens junctions. This article systematically explores the unique features, similarities, interplay, and clinical relevance of tight junctions versus adherens junctions in maintaining gut barrier integrity.
Table of Contents
- Introduction: The Intestinal Barrier and Its Junctional Complex
- Structural Overview of Junctional Complexes
- Tight Junctions: Architecture and Role
- Adherens Junctions: Organization and Function
- Key Differences Between Tight and Adherens Junctions
- Interplay and Regulation Between Tight and Adherens Junctions
- Regulation of Junctional Complexes in Gut Homeostasis
- Disruption of Junctions: Implications in Disease
- Therapeutic Perspectives and Future Directions
- Frequently Asked Questions (FAQs)
Introduction: The Intestinal Barrier and Its Junctional Complex
The intestinal barrier is a dynamic physiological structure that separates the internal milieu from a continually changing and potentially hostile external environment, including dietary antigens, microorganisms, and toxins. At its core lies the epithelial cell layer joined through specialized intercellular junctions:
- Tight junctions (also called zonula occludens)
- Adherens junctions (also called zonula adherens)
- Desmosomes (provide structural support)
These structures form the tripartite junctional complex unique to mammalian intestinal epithelium. Tight junctions seal the paracellular space, while adherens junctions provide strong mechanical attachment and influence signaling pathways essential for barrier maintenance.
Structural Overview of Junctional Complexes
| Junction Type | Main Protein Components | Location (relative to lumen) | Primary Function |
|---|---|---|---|
| Tight Junctions | Claudins, occludin, JAMs, ZO-1/-2/-3 | Most apical (closest to lumen) | Seal the intercellular space, regulate paracellular permeability, maintain cellular polarity |
| Adherens Junctions | E-cadherin, catenins, nectins, afadin | Just basal to tight junctions (slightly lower) | Mediate strong cell–cell adhesion, connect actin cytoskeleton, regulate signaling and morphogenesis |
Tight Junctions: Architecture and Role
Tight junctions form the most apical intercellular seal. These are multi-protein complexes adhering adjacent cell membranes so closely (eliminating the intercellular space) that they restrict the free passage of even small molecules between cells.
Principal protein families include:
- Claudins: Form the backbone of tight junction strands, dictate junction permeability properties.
- Occludin: Modulates junction integrity and interacts with other scaffolding proteins.
- Junctional Adhesion Molecules (JAMs): Regulate leukocyte transmigration and tight junction assembly.
- Zonula Occludens (ZO-1, ZO-2, ZO-3): Cytoplasmic scaffolding proteins link transmembrane proteins to the actin cytoskeleton and coordinate junctional assembly.
Key functions of tight junctions in the gut:
- Establish a selective barrier (paracellular gate) restricting non-specific solute and water leakage
- Maintain cell polarity by separating apical and basolateral membrane domains
- Enable dynamic regulation in response to physiological demands (absorption, secretion, immune activation)
Disrupted tight junction integrity leads to increased intestinal permeability (“leaky gut”) and heightened risk of inflammation or disease.
Adherens Junctions: Organization and Function
Adherens junctions are protein clusters on the lateral surfaces of epithelial cells, situated just below tight junctions. Unlike tight junctions, they do not create a sealed paracellular barrier; instead, they mediate robust intercellular adhesion through direct protein-protein interactions:
- E-cadherin: A type-I transmembrane glycoprotein making homophilic (like-with-like) contacts with cadherins on neighboring cells in a calcium-dependent fashion.
- Catenins (β-, γ-, p120-catenin): Intracellular adaptor proteins linking cadherin to the actin cytoskeleton. This linkage is critical for mechanical strength and cell signaling.
- Nectins and Afadin: Immunoglobulin-like adhesion molecules and an F-actin-binding protein that further stabilize the adherens junction and link to additional cytoskeletal components.
Principal roles of adherens junctions:
- Maintain strong cell–cell adhesion to preserve tissue architecture under mechanical stress
- Serve as key organizers of apical–basal polarity and regulators of tight junction assembly
- Coordinate cellular communication, epithelial proliferation, migration, and wound repair
E-cadherin downregulation is associated with weakened barrier, increased cell motility, and pathological conditions such as cancer and inflammatory bowel disease (IBD).
Key Differences Between Tight and Adherens Junctions
| Aspect | Tight Junctions | Adherens Junctions |
|---|---|---|
| Position | Most apical (topmost) | Basal to tight junctions |
| Physical Seal | Forms a nearly impermeable seal eliminating intercellular space | Leaves a ~20 nm gap between cells |
| Main Proteins | Claudins, occludin, JAMs, ZO proteins | E-cadherin, catenins, nectins, afadin |
| Main Function | Regulate paracellular permeability, barrier formation | Mechanical adhesion, cell polarity, initiation of tight junction assembly |
| Connection to Cytoskeleton | Anchored to actin via ZO proteins | Direct and indirect connections to actin via catenins and afadin |
Interplay and Regulation Between Tight and Adherens Junctions
Though often studied separately, tight junctions and adherens junctions are intimately linked both structurally and functionally:
- Adherens junction formation is required for the correct localization and assembly of tight junction components on the apical surface.
- Disruption of adherens junctions (e.g., loss of E-cadherin or catenins) can directly destabilize tight junctions, increasing paracellular permeability and decreasing barrier integrity.
- Signaling pathways involved in actin cytoskeleton remodeling (such as Rho GTPases and myosin light chain kinase) simultaneously affect both junction types.
- Tight junction assembly can in turn modulate adherens junction stability through reciprocal signaling, underscoring the dynamic cross-talk necessary for sustained barrier function.
This coordinated interaction ensures the intestinal barrier is both robust and capable of rapid adaptation to inflammatory, nutritional, or mechanical stimuli.
Regulation of Junctional Complexes in Gut Homeostasis
Junctional protein expression and function are precisely regulated by:
- Transcriptional control: Genes encoding claudins, occludin, JAMs (tight junctions) and E-cadherin, catenins (adherens junctions) are tightly modulated in response to developmental cues, cellular differentiation, and environmental stress.
- Post-translational modifications: Phosphorylation of junctional proteins can enhance or destabilize complexes, altering barrier properties.
- Cytokine signaling: Pro-inflammatory cytokines (TNFα, IFN-γ, IL-4, IL-13) can redistribute or degrade junctional proteins, markedly increasing intestinal permeability.
- AMP-activated protein kinase (AMPK): Activation of AMPK enhances tight junction assembly and barrier function, while its inhibition exacerbates permeability and inflammation.
- Actin cytoskeleton reorganization: Pathways that regulate actin dynamics (e.g., RhoA/ROCK, myosin light chain kinase) directly impact both junction types, facilitating barrier adaptation during injury or cell renewal.
Modulation of these pathways is essential for balancing gut absorption with immune defense, and maladaptive changes can trigger gut barrier dysfunction.
Disruption of Junctions: Implications in Disease
Compromised tight or adherens junctions are central in the pathogenesis of several gastrointestinal diseases:
- Inflammatory Bowel Disease (IBD): Increased cytokine production leads to altered expression and localization of ZO-1, occludin, and claudins, as well as disruption of E-cadherin-mediated adhesion, resulting in heightened permeability and inflammation.
- Celiac Disease: Dysregulated tight junctions account for increased solute leak and inflammation in response to gluten exposure.
- Infection (e.g., by enteric pathogens): Many bacteria produce toxins that target tight junction proteins, increasing paracellular permeability.
- Metabolic Syndrome and Obesity: Dysbiosis-related inflammation may compromise junction integrity, increasing translocation of bacterial products and perpetuating metabolic endotoxemia.
- Cancer: Downregulation or mutation of adherens junction proteins (notably E-cadherin) is a hallmark of epithelial-to-mesenchymal transition (EMT) in colorectal carcinoma.
Therapeutic Perspectives and Future Directions
- Targeting cytokine signaling or AMPK pathways offers potential to restore tight junction integrity and gut barrier function in inflammatory conditions.
- Small-molecule inhibitors and biological therapies are in development to reinforce junctional adhesion and reduce gut permeability.
- Dietary modulation (prebiotics, micronutrients) and probiotics may indirectly support barrier function by reducing inflammation and promoting epithelial health.
- Future research is exploring the dynamic regulation of junctional complexes during injury, regeneration, and chronic disease, with the hope of new diagnostic and therapeutic targets.
Frequently Asked Questions (FAQs)
Q: What is the most important function of tight junctions in the gut?
A: Tight junctions seal the space between adjacent epithelial cells, providing a selective barrier that prevents leakage of gut contents into surrounding tissue while regulating nutrient and water absorption.
Q: How are adherens junctions different from tight junctions?
A: Adherens junctions mediate strong mechanical adhesion between cells via E-cadherin–catenin complexes and are not completely sealing, while tight junctions create an effective paracellular barrier by fusing cell membranes at the apical surface.
Q: Can inflammation break down both tight and adherens junctions?
A: Yes, pro-inflammatory cytokines such as TNF-α and IFN-γ can cause redistribution or degradation of both types of junctional proteins, resulting in impaired barrier function and increased permeability .
Q: How do these junctions interact during tissue repair in the gut?
A: During epithelial repair, adherens junctions often form first, providing a scaffold for tight junction assembly. The dynamic interplay between these complexes orchestrates barrier reformation and regeneration.
Q: Are there therapies that can restore the gut barrier?
A: Strategies include targeting inflammation, supporting AMPK signaling, modulating the gut microbiome, and developing drugs that stabilize junctional proteins. Clinical applications are under active investigation.
References
Read full bio of medha deb












