Short-Chain Fatty Acids and Blood-Brain Barrier Integrity: The Critical Role of Butyrate in Neuroprotection and Gut-Brain Axis
A gut-derived metabolite activates protective pathways that preserve neural resilience.
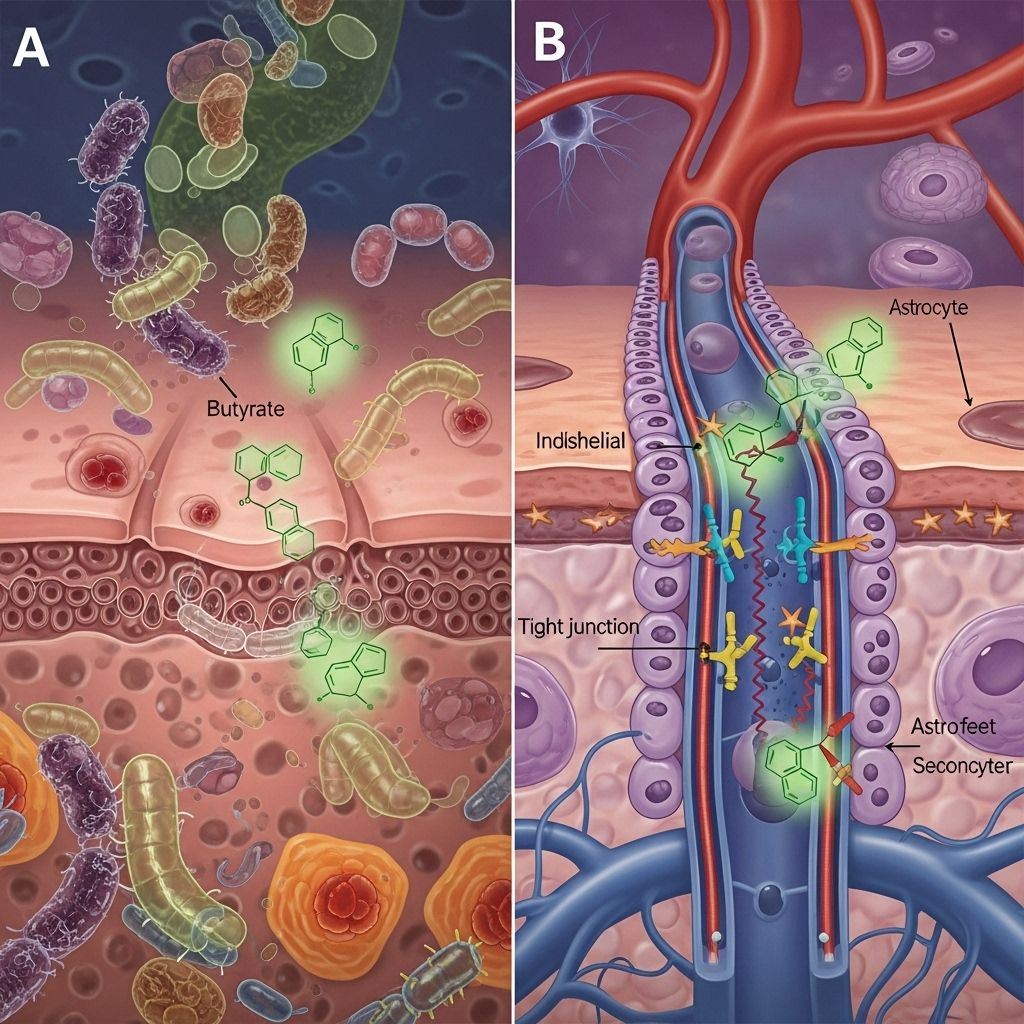
The discovery that metabolites produced by the gut microbiota can influence the central nervous system represents a paradigm shift in neuroscience and medicine. Among these metabolites, short-chain fatty acids (SCFAs)—particularly butyrate—have emerged as fundamental mediators of gut-brain communication. Butyrate exerts a profound effect on the blood-brain barrier (BBB), impacting both its structure and function and thereby influencing brain health.
Table of Contents
- Introduction to SCFAs and the Blood-Brain Barrier
- SCFA Production and Profiles
- The Blood-Brain Barrier: Structure and Significance
- Mechanisms of Blood-Brain Barrier Disruption in Neurological Disorders
- How Butyrate Maintains and Repairs BBB Integrity
- Molecular Mechanisms: Epigenetic and Signaling Pathways
- Evidence from Experimental and Clinical Studies
- Consequences of SCFA Dysregulation and BBB Vulnerability
- Therapeutic Potential and Future Directions
- Frequently Asked Questions (FAQs)
Introduction to SCFAs and the Blood-Brain Barrier
Short-chain fatty acids (SCFAs) are a class of organic acids comprising fewer than six carbon atoms, with acetate, propionate, and butyrate being the most prevalent in the human body. These molecules are produced predominantly in the colon through microbial fermentation of dietary fiber that escapes digestion in the upper gastrointestinal tract .
Alongside their well-characterized local roles in gut health and metabolism, SCFAs have demonstrated systemic effects, including acting as key mediators of the gut-brain axis. Notably, SCFAs can:
- Modulate immune responses and inflammation
- Provide energy to colonocytes and distant tissues
- Signals through G-protein-coupled receptors (GPCRs)
- Influence gene expression via epigenetic mechanisms such as histone deacetylase (HDAC) inhibition
A particularly significant function is their role in maintaining the integrity of the blood-brain barrier, a crucial interface between the systemic circulation and the central nervous system .
SCFA Production and Profiles
SCFA production occurs primarily in the large intestine thanks to the activity of gut microbiota, particularly bacteria in the Firmicutes and Bacteroidetes phyla. The major SCFAs ( acetate, propionate, and butyrate) are produced in approximately a 60:25:15 distribution in humans .
- Dietary fiber—notably resistant starches, inulin, and certain oligosaccharides—is the chief substrate for SCFA production.
- Butyrate is primarily synthesized by bacterial genera such as Faecalibacterium, Eubacterium, and Roseburia.
- Acetate and propionate are produced by various Bacteroidetes and other Firmicutes.
Upon production, SCFAs are absorbed by colonocytes or enter the portal blood, where they exert local effects or travel to distant organs, including the brain .
The Blood-Brain Barrier: Structure and Significance
The blood-brain barrier (BBB) is a highly selective semipermeable border that separates circulating blood from the brain’s extracellular fluid. Comprised primarily of endothelial cells joined by tight junction proteins, as well as pericytes, astrocyte end-feet, and the basement membrane, the BBB fulfills several essential functions:
- Protects the brain from pathogens and toxins in the bloodstream
- Regulates the transport of nutrients, gases, and metabolic waste
- Maintains the ionic and molecular homeostasis critical for neuronal signaling
Disruption or compromise of BBB integrity is a hallmark of numerous neurological and neurodegenerative disorders, making its maintenance vital for brain health .
Mechanisms of Blood-Brain Barrier Disruption in Neurological Disorders
BBB breakdown is implicated in multiple conditions, such as:
- Neuroinflammation (e.g., multiple sclerosis, sepsis-associated encephalopathy)
- Neurodegeneration (e.g., Alzheimer’s disease, Parkinson’s disease)
- Acute brain injuries (e.g., hypoxia-ischemia, traumatic brain injury)
- Aging-related BBB impairment
Central to these pathologies is the disturbance of intercellular tight junction complexes—proteins such as claudins, occludins, and junctional adhesion molecules—which results in increased vascular permeability and entry of peripheral inflammatory mediators into the brain .
How Butyrate Maintains and Repairs BBB Integrity
Butyrate stands out as a major SCFA with direct and indirect actions on the BBB:
- Reduces BBB permeability by restoring or increasing expression of critical tight junction proteins such as claudin-5 and occludin
- Promotes anti-inflammatory and anti-oxidative responses in brain endothelial cells
- Suppresses pro-inflammatory cytokine production—reducing microglial reactivity and protecting neural tissues
- Significantly, butyrate can directly cross the BBB via monocarboxylate transporters (MCTs), reaching local concentrations sufficient to induce physiological effects
Animal studies and in vitro models show that butyrate supplementation promotes recovery of BBB function in models of brain injury, neuroinflammation, and neurodegeneration .
Molecular Mechanisms: Epigenetic and Signaling Pathways
The neuroprotective effects of butyrate on the BBB are mediated through multiple, sometimes overlapping, mechanisms:
- Histone Deacetylase Inhibition (HDACi): Butyrate is a potent inhibitor of HDACs, enzymes that modulate gene expression by altering chromatin accessibility. Inhibition fosters anti-inflammatory and antioxidant gene expression, restoring junction protein transcription and reducing BBB permeability .
- Activation of G-Protein-Coupled Receptors (GPCRs): SCFAs signal through GPCRs like FFAR2 (GPR43) and FFAR3 (GPR41), initiating cascades that influence inflammation, metabolism, and barrier integrity.
- NF-κB and Nrf2 Pathway Modulation: Butyrate downregulates pro-inflammatory NF-κB signaling while upregulating antioxidant Nrf2 pathways, further enhancing BBB protection .
- Direct Effects on Tight Junction Proteins: Butyrate preserves or restores localization and expression of claudin-5, occludin, and ZO-1 in brain endothelial cells.
| Mechanism | Functional Outcome |
|---|---|
| HDAC inhibition | Anti-inflammatory gene expression, restoration of tight junctions |
| GPCR activation (FFAR2, FFAR3) | Anti-inflammatory, metabolic regulation, barrier protection |
| NF-κB downregulation, Nrf2 upregulation | Reduced inflammation, increased antioxidant defense |
| Direct upregulation of BBB proteins | Greater barrier tightness, reduced permeability |
Evidence from Experimental and Clinical Studies
Accumulating evidence from a range of scientific approaches underscores the protective role of butyrate in BBB maintenance:
- Germ-free animal studies: Mice lacking microbiota (and thus gut-derived SCFAs) display increased BBB permeability; colonization or supplementation with butyrate reverses this deficit .
- In vitro endothelial cell models: Treatment with butyrate upregulates tight junction protein expression and decreases paracellular permeability .
- In vivo CNS disease models: Oral or parenteral butyrate lessens BBB disruption and reduces neuroinflammation in conditions such as hypoxic-ischemic injury, sepsis-associated encephalopathy, and Parkinson’s disease .
- Human implications: While most direct evidence is preclinical, several studies associate low butyrate-producing gut bacteria and reduced SCFA levels with cognitive decline and neurodegenerative diseases.
Consequences of SCFA Dysregulation and BBB Vulnerability
Disturbances in gut microbiota—arising from antibiotic use, poor diet, or disease—often lead to a drop in butyrate production. This dysregulation weakens the BBB and may:
- Increase the brain’s vulnerability to pathogens and inflammatory mediators
- Promote neuroinflammation
- Accelerate neurodegeneration in susceptible individuals
- Contribute to the pathogenesis of aging-related cognitive decline
Restoring SCFA equilibrium with dietary fiber, probiotics, or direct butyrate supplementation is now a major focus of neurotherapeutics and preventive medicine .
Therapeutic Potential and Future Directions
Targeting the gut microbiota–SCFA–BBB axis is a promising approach to promoting brain health and treating neurological diseases. Strategies include:
- Increased dietary fiber intake to stimulate endogenous butyrate production
- Probiotic or next-generation microbial therapeutics to boost butyrate-producing taxa
- Direct use of butyrate or related HDAC inhibitors as adjuncts to therapy
- Personalized nutrition and microbiome profiling to identify at-risk individuals and optimize interventions
However, challenges remain. The complexity of the gut microbial ecosystem and inter-individual variability can affect treatment outcomes. Standardizing therapeutic agents and translating preclinical findings to robust human trials is ongoing .
Frequently Asked Questions (FAQs)
Q: What are short-chain fatty acids, and how are they produced?
A: SCFAs are organic acids with fewer than six carbon atoms, mainly acetate, propionate, and butyrate, produced by gut bacteria fermenting dietary fiber in the colon.
Q: How does butyrate reach the brain and influence the BBB?
A: Butyrate crosses the blood-brain barrier via monocarboxylate transporters expressed on endothelial cells and modulates barrier integrity by upregulating tight junction proteins, reducing inflammation, and acting as an epigenetic regulator.
Q: Can altering the gut microbiota improve BBB health?
A: Yes, interventions like high-fiber diets, prebiotics, probiotics, and direct butyrate supplementation can increase SCFA production and have been shown in preclinical studies to restore BBB integrity.
Q: What neurological diseases are associated with BBB breakdown related to SCFA deficiency?
A: BBB breakdown due to low SCFA (especially butyrate) levels has been implicated in conditions such as multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and acute CNS injuries.
Q: Are there any clinical trials using butyrate to treat neurological disorders?
A: While the majority of evidence is preclinical, there is growing interest in butyrate and related therapies for brain health; however, large-scale human clinical trials are still in early stages.
References
Read full bio of medha deb












