H. Pylori Infection and Gastric Barrier Integrity: Mechanisms, Disruption, and Clinical Implications
Uncover the molecular tactics bacteria use to breach stomach defenses and spark disease.
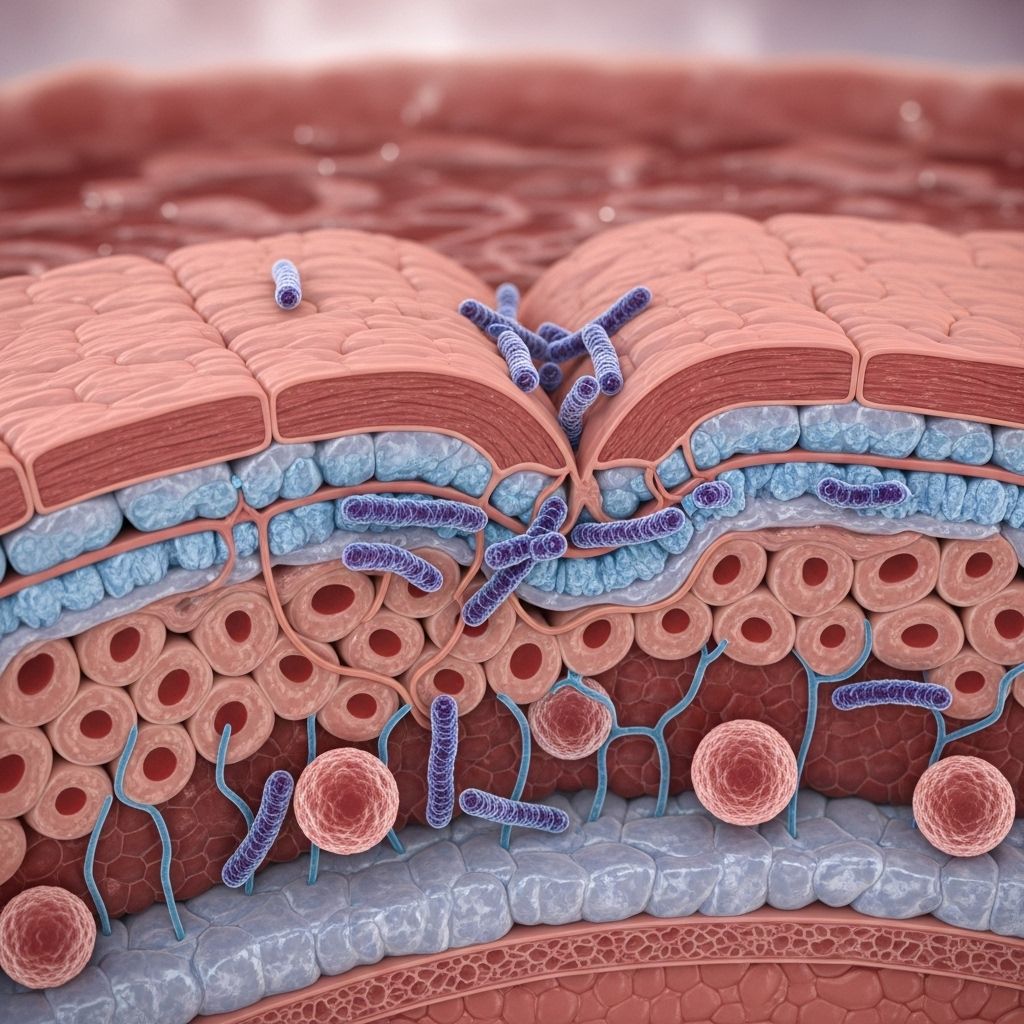
Helicobacter pylori (H. pylori) is a spiral-shaped bacterium that colonizes the human gastric mucosa, affecting over half the global population. While infection can be asymptomatic, it is strongly associated with the development of chronic gastritis, peptic ulcer disease, and gastric cancer. A major pathological consequence is the disruption of the gastric barrier integrity, the fundamental defense that protects the stomach lining from its harsh acidic environment and pathogens. This article examines how H. pylori interacts with and compromises the gastric barrier, the molecular and cellular mechanisms involved, and the broader clinical implications.
Table of Contents
- Overview of the Gastric Barrier
- Introduction to Helicobacter pylori Infection
- Mechanisms by which H. pylori Disrupts Gastric Barrier Integrity
- Inflammatory and Immune Responses
- Clinical Consequences of Barrier Disruption
- Diagnosis and Assessment of Barrier Integrity in H. pylori Infection
- Therapeutic Approaches
- Recent Advances and Future Perspectives
- Frequently Asked Questions
Overview of the Gastric Barrier
The gastric barrier is a multilayered defense system comprising the following components:
- Mucus-bicarbonate layer: Secreted by surface mucous cells, this gel neutralizes acid near the epithelial surface.
- Epithelial cell layer: Connected by tight junctions and adherens junctions, these cells provide a physical barrier.
- Surface renewal and repair: The rapid turnover of epithelial cells promotes healing and prevents prolonged injury.
- Immune surveillance: Subjacent immune cells recognize pathogens and modulate inflammatory responses.
Together, these systems guard against acid, mechanical injury, and microbial invasion, maintaining gastric homeostasis.
Introduction to Helicobacter pylori Infection
H. pylori is a Gram-negative, microaerophilic bacterium adapted to survive in the acidic stomach environment. Transmission is typically oral-oral or fecal-oral and often occurs during childhood. While many infections remain asymptomatic, a subset of individuals develop chronic gastritis, peptic ulcers, and gastric malignancies.
Key features facilitating H. pylori colonization include:
- Production of urease to neutralize local acidity
- Motility via flagella to navigate mucus
- Expression of adhesins for gastric epithelial attachment
- Secretion of virulence factors (e.g., CagA, VacA, HtrA)
Mechanisms by which H. pylori Disrupts Gastric Barrier Integrity
The Role of Gastric Acidity in Barrier Function
Gastric acidity itself strengthens the barrier by tightening epithelial junctions. Under normal conditions, increased acidity leads to:
- Increased transepithelial electrical resistance (TEER)
- Decreased paracellular permeability
However, H. pylori diminishes this acid-induced tightening in a urease-dependent manner. By producing ammonia through urease activity, H. pylori elevates the local pH, countering protective acid effects, loosening junctions, and compromising the epithelial barrier’s integrity. This weakens defense and facilitates mucosal damage and ulcer formation.
Molecular Disruption of Tight and Adherens Junctions
Central to barrier compromise is the direct targeting of tight junction (TJ) and adherens junction (AJ) proteins by H. pylori and its virulence factors:
- CagA (cytotoxin-associated gene A): Once injected into epithelial cells via a Type IV secretion system, CagA binds to TJ proteins such as ZO-1 and JAM, redistributes them, and disrupts cell polarity. This can lead to leaky epithelia and increased permeability.
- CagA also inhibits kinases such as PAR1 and interferes with PKCα, resulting in further polarity and junctional defects.
- Interaction with actin cytoskeleton components (e.g., Par1b/ZO-1) aggravates cellular depolarization.
- HtrA (high-temperature requirement A protease): HtrA degrades E-cadherin and other junctional proteins, opening intercellular spaces for bacterial invasion.
- Other factors, such as Rho kinase activation and tight junction protein-4 degradation, further weaken the barrier.
Overall, H. pylori prompts a structural reorganization of the epithelial barrier, increasing the risk of hyperpermeability and disease progression.
| Factor | Target | Effect |
|---|---|---|
| CagA | ZO-1, JAM, PAR1, PKCα | Disrupts junctions, cell polarity, increases permeability |
| VacA | Mitochondria, cellular membrane | Induces apoptosis, damages cells |
| HtrA | E-cadherin, claudins | Cleaves junction proteins, enables bacterial entry |
| Urease | Gastric mucus | Neutralizes acid, disrupts acid-induced barrier tightening |
Induction of Apoptosis in Epithelial Cells
Another critical mechanism is the promotion of apoptosis (programmed cell death) of gastric epithelial cells. H. pylori, via toxins such as VacA, urease, and persistent inflammation, leads to sustained epithelial cell loss.
- Accelerated apoptosis disrupts the cell layer, exposing underlying tissue to acid, pepsin, and bacteria.
- An imbalance between apoptosis and regeneration weakens the mucosal barrier.
- Immune factors, such as TNF-α and IFN-γ, produced during infection, amplify apoptotic pathways.
This apoptotic turnover makes the gastric mucosa more susceptible to injury, ulceration, and transformation to preneoplastic states.
Inflammatory and Immune Responses
The persistent presence of H. pylori stimulates an active inflammatory response involving epithelial cells, neutrophils, macrophages, and lymphocytes.
- Cytokine release: H. pylori alters cytokine release profiles. In the presence of acid, uninfected epithelia reduce interleukin-8 (IL-8, a chemokine attracting neutrophils) but increase IL-6 (pro-inflammatory cytokine) secretion. Infection disrupts these normal responses, often resulting in uncoordinated inflammation.
- Innate immune signaling: H. pylori manipulates pathways such as the cGAS-STING axis and type I interferon signaling, both in immune and epithelial cells. This can result in aberrant DNA sensing and promote metaplastic changes.
- Free radicals and oxidative stress: Infiltrating neutrophils generate reactive oxygen species (ROS) that damage epithelial DNA and further weaken barrier function.
Altogether, these processes lead to chronic gastritis, mucosal erosion, and, over time, predispose to ulcer and neoplastic transformation.
Clinical Consequences of Barrier Disruption
Disrupted gastric barrier integrity due to H. pylori infection underlies several important diseases:
- Chronic gastritis: Resulting from persistent inflammation and cell injury.
- Peptic ulcer disease: Due to impaired barrier allowing acid and pepsin-induced mucosal injury.
- Intestinal metaplasia and dysplasia: Ongoing injury, apoptosis, and metaplastic signaling promote preneoplastic changes.
- Gastric carcinoma: Chronic inflammation, genetic mutations, and cellular disorganization foster malignant transformation.
Diagnosis and Assessment of Barrier Integrity in H. pylori Infection
The gold standard for diagnosing H. pylori infection is endoscopic biopsy with histological examination and special staining. Non-invasive options include:
- Urea breath tests
- Stool antigen tests
- Serologic testing for antibodies (less specific for active infection)
Gastric barrier integrity is assessed indirectly in the clinic by endoscopic findings of erosions or ulcers, as well as by measuring gastric permeability or loss of junctional proteins with immunohistochemistry in research settings.
Therapeutic Approaches
The mainstay of therapy is H. pylori eradication:
- Triple therapy: Proton pump inhibitor (PPI) plus two antibiotics
- Quadruple therapy: Includes bismuth and another antibiotic for resistant cases
Adjunctive therapies are directed toward restoring barrier function:
- PPIs reduce gastric acidity, promoting mucosal healing
- Probiotics may help restore mucosal defense
- Antioxidants can mitigate ROS-induced injury
Future research is focused on targeted agents that disrupt bacterial adherence or directly stabilize junctional complexes.
Recent Advances and Future Perspectives
- Molecular understanding: Ongoing research further delineates the interplay between bacterial effector proteins, host cell signaling, and structural proteins of the gastric barrier.
- Biomarkers: Identification of new biomarkers for barrier dysfunction and disease risk is an area of rapid development.
- Therapeutic innovation: Development of novel strategies to specifically block H. pylori virulence mechanisms or enhance barrier repair are ongoing.
Frequently Asked Questions (FAQs)
How does H. pylori survive the acidic environment of the stomach?
The bacterium produces the enzyme urease, which converts urea to ammonia, neutralizing acid locally and enabling colonization of the mucus layer.
What are the main structural components of the gastric barrier affected by H. pylori?
Tight junctions (ZO-1, claudins), adherens junctions (E-cadherin), and the apical cell membrane are the primary structures disrupted, leading to increased permeability and tissue exposure.
How does apoptosis contribute to gastric barrier dysfunction in H. pylori infection?
Apoptosis increases cell shedding and disrupts the epithelial lining. If regeneration fails to keep pace, the barrier weakens, promoting ulceration and inflammation.
Is barrier restoration possible after eradication of H. pylori?
In many cases, removal of the infection allows regeneration of the gastric epithelium and reestablishment of normal barrier function, especially if caught before advanced atrophy or transformation have occurred.
What are the long-term risks if H. pylori infection is left untreated?
Chronic infection increases the risk of peptic ulcers, persistent gastritis, mucosal atrophy, intestinal metaplasia, and gastric adenocarcinoma.
References
- https://pubmed.ncbi.nlm.nih.gov/23989011/
- https://pmc.ncbi.nlm.nih.gov/articles/PMC4177462/
- https://www.frontiersin.org/journals/cellular-and-infection-microbiology/articles/10.3389/fcimb.2025.1601501/full
- https://www.dovepress.com/helicobacter-pylori-induced-apoptosis-in-gastric-diseases-mechanisms-i-peer-reviewed-fulltext-article-IJGM
Read full bio of medha deb












