Endotoxin (LPS) as a Central Driver of Systemic Barrier Dysfunction and Chronic Disease Pathogenesis
Gut barrier breaches allow toxins to undermine defenses, sparking silent immune activation.
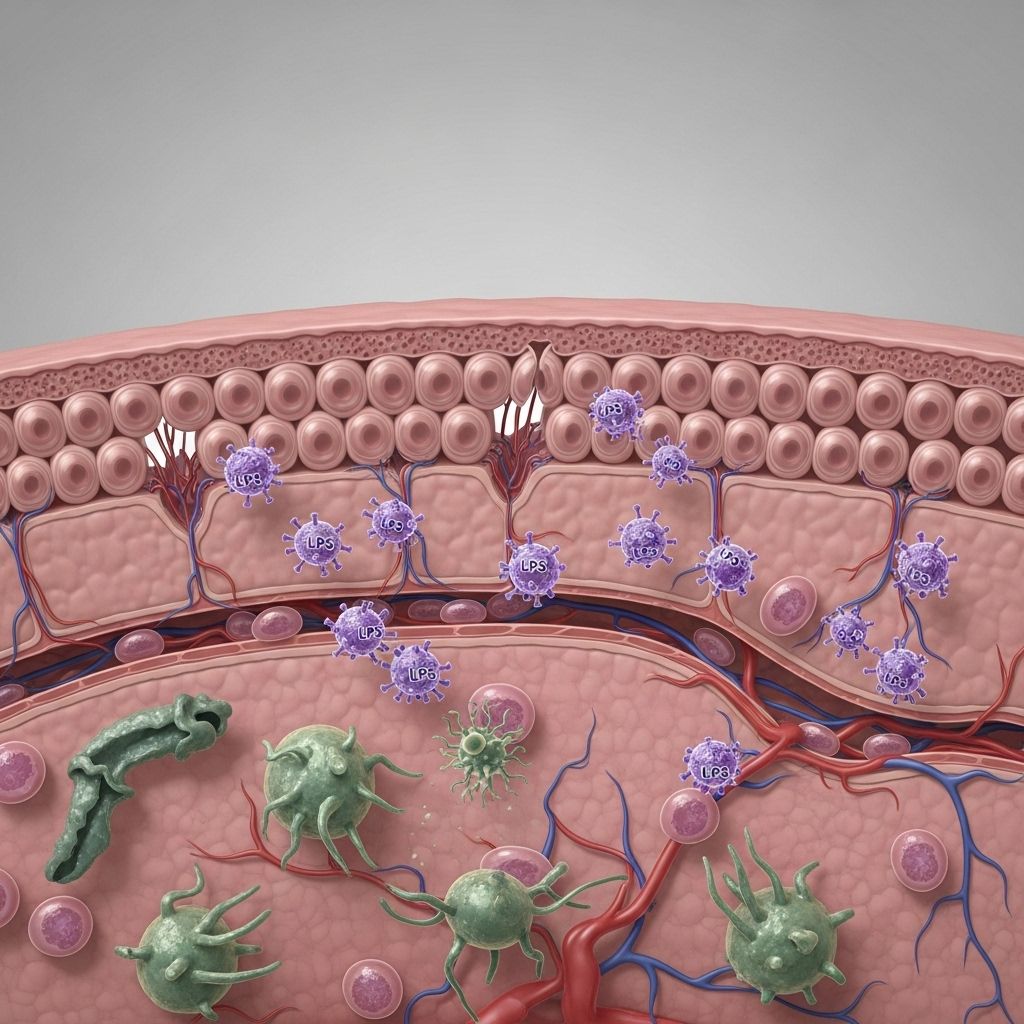
Lipopolysaccharide (LPS), also known as endotoxin, is a critical molecule linking gut health, immune activation, and the onset of chronic systemic diseases. Disruption of epithelial barriers by LPS triggers inflammation and systemic dysfunction, driving progressive pathology in organs far beyond the gut itself.
Table of Contents
- Introduction to Endotoxin and Barrier Dysfunction
- Structure and Source of Endotoxin (LPS)
- The Intestinal Barrier System: Layers and Functions
- Mechanisms of LPS Translocation Across Barriers
- Systemic Effects of LPS on Host Barriers
- Immune Activation, Inflammation, and Disease
- Links Between LPS, Barrier Dysfunction, and Chronic Disease
- Assessment of Barrier Dysfunction: Biomarkers and Diagnostics
- Therapeutic Strategies to Restore Barrier Integrity
- Frequently Asked Questions (FAQs)
- Conclusion
Introduction to Endotoxin and Barrier Dysfunction
The human body relies on a series of physical and biochemical barriers—such as the intestinal, respiratory, blood-brain, and vascular endothelia—to prevent the passage of pathogens and toxins from the environment into systemic circulation. However, when these barriers are compromised, molecules like lipopolysaccharide (LPS) can translocate from their bacterial sources into the bloodstream, initiating systemic immune reactions that underpin many chronic diseases.
Structure and Source of Endotoxin (LPS)
Endotoxin refers to LPS, a complex glycolipid component of the outer membrane of Gram-negative bacteria. LPS consists of three domains:
- Lipid A: The biologically active, hydrophobic anchor responsible for much of LPS’s pro-inflammatory activity.
- Core oligosaccharide: A central region of branched sugars.
- O-antigen: Repeating sugar residues extending outwards, varying among bacterial species.
LPS is considered a prototypical pathogen-associated molecular pattern (PAMP), triggering vertebrate immune systems through specialized receptors such as TLR4. The main source of LPS exposure in humans is the gut microbiota, particularly the highly abundant Gram-negative bacteria in the large intestine.
The Intestinal Barrier System: Layers and Functions
The intestinal barrier consists of several interconnected layers that serve to restrict the passage of harmful substances, while permitting nutrient absorption:
- Mucus layer: The first defense, containing mucins and antimicrobial factors.
- Epithelial cell layer: Comprised of tightly connected epithelial cells (with tight junctions, adherens junctions, and desmosomes).
- Immune cells (lamina propria): Immune surveillance by lymphocytes, dendritic cells, and macrophages.
- Secretory IgA: Immunoglobulin A neutralizes antigens and toxins in the lumen.
When any layer of this defense fails, LPS and other microbial metabolites can cross into circulation—a process sometimes termed “leaky gut” or intestinal barrier dysfunction.
Key Barrier Components and Their Role in Disease Prevention
| Barrier Component | Function | Impact of Impairment |
|---|---|---|
| Mucus Layer | Physical trap for microbes/toxins, contains antimicrobial peptides | Increased contact of bacteria with epithelium; higher inflammation risk |
| Epithelial Cells | Structural restraint; tight junctions regulate transit | Paracellular leakage of LPS and toxins |
| Immune Layer (Lamina Propria) | Immune cell detection and control of pathogens | Overstimulation, chronic immune activation |
| sIgA | Immune exclusion via luminal neutralization | Increased antigen/toxin penetration |
Mechanisms of LPS Translocation Across Barriers
For LPS to drive systemic dysfunction, it must cross epithelial barriers and enter the circulation. Several mechanisms facilitate LPS translocation:
- Disruption of Tight Junctions: LPS and inflammatory cytokines disrupt proteins like ZO-1 and occludin, creating intercellular leaks.
- Bacterial Overgrowth and Dysbiosis: Increased Gram-negative populations elevate the intestinal LPS pool.
- Dietary Factors: High-fat, Western diets alter microbiota and increase LPS absorption, sometimes via chylomicron-facilitated pathways.
- Physical Stress and Injury: Ischemia, infection, or trauma can break down the mucus and epithelial integrity.
Once in blood, most LPS is bound by LPS-binding protein (LBP) and shuttled to CD14 and TLR4—the main immune-activating machinery.
Systemic Effects of LPS on Host Barriers
The presence of LPS in systemic circulation (endotoxemia) is recognized as both an acute and chronic threat to body homeostasis:
- Acute Effects: High levels of LPS (as seen in sepsis) trigger a “cytokine storm,” leading to fever, shock, and potential multi-organ failure.
- Chronic Low-Grade Endotoxemia: Persistent, subclinical LPS exposure is associated with low-grade inflammation, insulin resistance, and progressive barrier deterioration in other organs (e.g., lung, liver, blood-brain barrier).
Activated resident and infiltrating macrophages in tissues respond to LPS by releasing cytokines (e.g., TNF-α, IL-1β, IL-6), perpetuating inflammation and tissue dysfunction.
Immune Activation, Inflammation, and Disease
LPS is detected by the innate immune system via the TLR4/MD2/CD14/LBP complex:
- LPS-LBP complex binds to CD14 (membrane and soluble forms).
- CD14 assists in presenting LPS to TLR4/MD2, triggering TLR4 dimerization and intracellular signaling mainly through the MyD88 pathway.
- This cascade activates NF-κB and AP-1 transcription factors, upregulating pro-inflammatory cytokines and acute-phase proteins (CRP, PGE2).
Summary of LPS-triggered cytokine production:
- Tumor necrosis factor-alpha (TNF-α)
- Interleukin-1 beta (IL-1β)
- Interleukin-6 (IL-6)
- Acute phase proteins (C-reactive protein)
At low concentrations, persistent LPS exposure maintains a “primed” inflammatory state—termed metabolic endotoxemia—which is now recognized as a key driver of chronic non-communicable diseases.
Links Between LPS, Barrier Dysfunction, and Chronic Disease
Increasing evidence implicates endotoxin-driven barrier dysfunction in a wide range of diseases:
- Metabolic disorders (Type 2 diabetes, obesity): LPS-induced inflammation in adipose tissue and liver leads to insulin resistance, promotes steatosis, and perpetuates metabolic syndrome.
- Cardiovascular disease: Chronic endotoxemia is associated with atherosclerotic plaque formation and vascular inflammation.
- Neurodegenerative diseases: LPS translocation may compromise the blood-brain barrier, contributing to neuroinflammatory processes seen in Parkinson’s and Alzheimer’s disease.
- Liver disease (NAFLD/NASH): Hepatic accumulation of LPS drives Kupffer cell activation and fibrogenesis.
- Chronic kidney disease, autoimmune diseases, and even cancer are linked to sustained LPS exposure and systemic inflammation.
Metabolic Endotoxemia vs. Sepsis: A Comparison
| Parameter | Metabolic Endotoxemia | Sepsis/Acute Endotoxemia |
|---|---|---|
| LPS Level | Low, chronic (<1 ng/mL) | High, acute (>10 ng/mL) |
| Symptoms | Subclinical, chronic inflammation | Fever, hypotension, organ failure |
| Disease Link | Obesity, T2DM, CVD, neurodegeneration | Septic shock, acute organ dysfunction |
| Mechanism | Barrier leakiness, metabolic/bacterial dysbiosis | Overwhelming infection |
Assessment of Barrier Dysfunction: Biomarkers and Diagnostics
Given the role of barrier dysfunction in disease, accurate assessment is crucial. Current and emerging approaches:
- Direct LPS quantification in blood (using LAL assay or mass spectrometry with limitations owing to interference by plasma proteins).
- LPS-binding protein (LBP) and soluble CD14 levels as surrogate markers for circulating LPS activity.
- Intestinal permeability tests (e.g., lactulose/mannitol absorption, FITC-dextran tracing).
- Markers of tight junction protein expression/function (e.g., ZO-1, occludins).
- Non-invasive assessment of fecal calprotectin, secretory IgA, or microbial metabolites as indirect indicators of barrier status.
Future developments may allow identification of which “layer” of the barrier is impaired, paving the way for precision therapeutic interventions.
Therapeutic Strategies to Restore Barrier Integrity
Given the causal link between LPS-driven barrier dysfunction and chronic disease, intervention strategies are under investigation:
- Nutritional approaches:
- Prebiotics (e.g., galactooligosaccharides) to boost beneficial bacteria and fortify mucus.
- Phytochemicals (e.g., curcumin) and vitamin D to enhance tight junction expression and function.
- Dietary adjustments, such as reducing saturated fat intake, to decrease Gram-negative overgrowth and improve epithelial health.
- Modulation of bile acids, which regulate microbial composition and intestinal permeability.
- Supplementation with endogenous enzymes (e.g., intestinal alkaline phosphatase, IAP) to neutralize LPS and protect the barrier.
- Novel therapeutics targeting TLR4 or its downstream inflammatory pathways.
Personalized treatment may become feasible as validated biomarkers allow specific identification of barrier “layer” defects.
Frequently Asked Questions (FAQs)
What is endotoxin (LPS) and why is it harmful?
LPS is a molecule from Gram-negative bacterial walls. When it escapes the gut and enters the bloodstream, it triggers potent inflammatory immune responses, implicated in the development of multiple chronic diseases.
What causes gut barrier dysfunction?
Factors include poor diet (high-fat/low-fiber), chronic stress, infections, medication use (such as NSAIDs), and genetic predispositions. These weaken the integrity of mucus, epithelial cells, and tight junctions, making it easier for LPS to translocate.
How can I test if I have barrier dysfunction?
Tests include lactulose/mannitol permeability assays, blood levels of LPS, LBP, and sCD14. Indirect indications can be gleaned from markers like fecal calprotectin and reduced sIgA.
Can barrier dysfunction and LPS-driven problems be reversed?
Restoring diet quality, promoting a healthy microbiome, supplementing with nutrients like fiber, vitamin D, or prebiotics, and in some cases using pharmaceutical agents, can help restore barrier integrity and reduce systemic LPS exposure.
Are all sources of LPS equally harmful?
The vast majority of circulating LPS is derived from the gut, but not all Gram-negative bacteria are equally pro-inflammatory. The structure of lipid A varies and influences the molecule’s inflammatory potential.
Conclusion
Bacterial endotoxin (LPS) is a central trigger of systemic immune activation when epithelial barriers are compromised. This ongoing “silent” inflammation is a major risk factor for a host of chronic diseases, including metabolic, cardiovascular, hepatic, and neurodegenerative conditions. Advances in diagnostics and precision therapies targeting specific barrier defects hold significant promise for mitigating the impact of LPS-driven pathologies and improving long-term health outcomes.
References
- https://pmc.ncbi.nlm.nih.gov/articles/PMC7033038/
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.594150/full
- https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2015.00223/full
- https://pubmed.ncbi.nlm.nih.gov/32099951/
- https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.29432
- https://www.nature.com/articles/s12276-018-0126-x
Read full bio of Sneha Tete












