Chemical Versus Physical Disruption of the Blood-Brain Barrier: Mechanisms, Risks, and Therapeutic Implications
Dual molecular and mechanical approaches bypass neural defenses for targeted therapy.
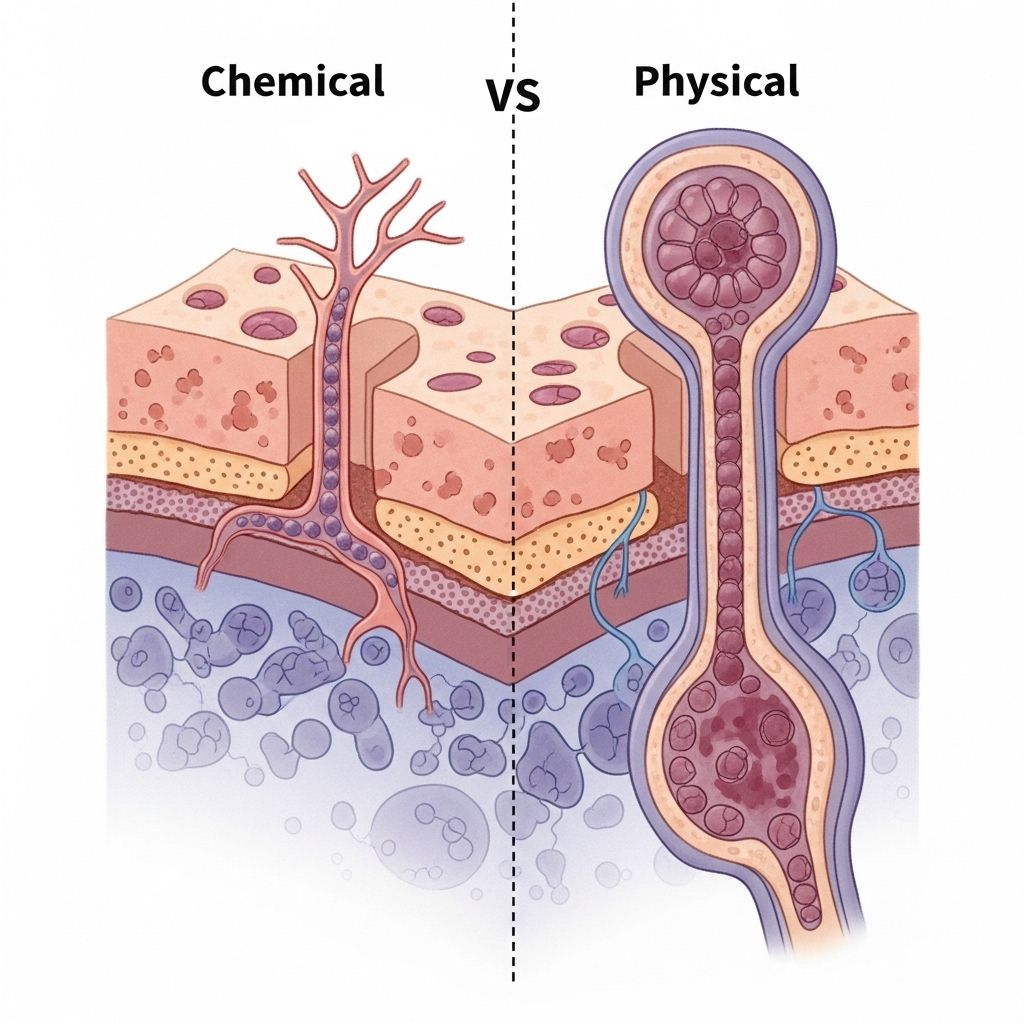
The blood-brain barrier (BBB) is a highly selective, semipermeable membrane that separates the circulating blood from the brain and central nervous system (CNS). While its primary function is to protect neural tissue from toxins, pathogens, and fluctuations in blood composition, the BBB poses significant challenges for drug delivery to treat CNS disorders. This article provides an extensive, comparative analysis of chemical versus physical BBB disruption methods, exploring underlying mechanisms, therapeutic applications, safety concerns, and future perspectives.
Table of Contents
- Introduction to the Blood-Brain Barrier
- Structure and Physiological Function of the Blood-Brain Barrier
- Rationale for BBB Disruption in Therapeutics
- Chemical Disruption of the Blood-Brain Barrier
- Physical Disruption of the Blood-Brain Barrier
- Comparative Analysis: Chemical vs Physical BBB Disruption
- Clinical Applications and Current Research
- Safety Concerns and Limitations
- Future Directions
- Frequently Asked Questions (FAQs)
Introduction to the Blood-Brain Barrier
The human brain relies on the BBB for its protection, which acts as a dynamic interface between the bloodstream and neural tissues. Although indispensable for health, the BBB restricts the delivery of therapeutic agents, creating a major bottleneck in CNS drug development. Consequently, researchers have developed a variety of strategies to disrupt the BBB temporarily and selectively, with two primary modalities: chemical and physical disruption.
Structure and Physiological Function of the Blood-Brain Barrier
The BBB is composed of specialized endothelial cells joined by tight junctions, a basement membrane, astrocytic end-feet, and pericytes. The barrier restricts molecules based on size, charge, and lipophilicity, and actively pumps out unwanted substances via efflux transporters. This unique architecture offers protection but also hinders pharmacologic interventions targeting the brain.
- Tight Junctions: Protein complexes sealing intercellular spaces and governing paracellular transport.
- Astrocytic End-Feet: Support structural integrity and control permeability.
- Pericytes: Regulate blood flow and contribute to barrier maintenance.
Rationale for BBB Disruption in Therapeutics
Most CNS diseases, including brain tumors, Alzheimer’s, and Parkinson’s disease, require pharmacologic agents to cross the BBB for effective therapy. Conventional drugs often lack sufficient penetration, leading to limited efficacy and increased systemic toxicity. Temporarily disrupting BBB integrity allows therapeutic molecules—ranging from small drugs to antibodies and gene vectors—to reach brain tissue.
Chemical Disruption of the Blood-Brain Barrier
Underlying Mechanisms
Chemical BBB disruption involves the administration of agents that modulate barrier permeability by inducing cellular or molecular changes in BBB structure. The most commonly used chemical method is hyperosmotic disruption, notably with mannitol.
- Hyperosmotic Agents: Intravenous (IV) or intra-arterial (IA) injections of hypertonic solutions (e.g., mannitol, arabinose) cause endothelial cells to shrink, increasing tensile stress at cell-cell junctions and resulting in transient opening of tight junctions.
- Biochemical Modulators: Bradykinin, histamine, and other vasoactive substances can alter junction protein expression or phosphorylation, increasing permeability.
- Inflammatory Mediators: Lipopolysaccharide (LPS) or cytokines induce inflammation, weakening the barrier but at the expense of broad, non-specific immune responses.
Key Features of Chemical Disruption
- Rapid onset: Transport increases almost instantaneously after dosing.
- Short duration: Permeability returns to baseline within minutes (typically 5–10 min) under controlled conditions.
- Non-selective: Affects both healthy and diseased tissue in the targeted vascular territory.
- Used for delivery of chemotherapeutics, antibodies, nanoparticles, and gene therapy vectors.
Clinical Example: Mannitol-Induced BBB Opening
| Agent | Mechanism | Duration | Applications |
|---|---|---|---|
| Mannitol | Osmotic cell shrinkage, tight junction disruption | 5–10 min | Chemotherapy for gliomas, delivery of antibodies and nanoparticles |
Advantages and Limitations
- Advantages: Simplicity, rapid action, cost-effectiveness; familiar to clinicians.
- Limitations: Non-specific disruption can risk neurotoxicity, edema, or unwanted entry of harmful substances; reproducibility issues have limited widespread clinical adoption.
Physical Disruption of the Blood-Brain Barrier
Underlying Mechanisms
Physical BBB disruption methods employ energy-based modalities to temporarily and locally increase permeability, often with high spatial and temporal control. Major physical approaches include:
- Focused Ultrasound (FUS): Uses acoustic waves often combined with intravenous microbubbles. Cavitation of these microbubbles generates mechanical stress, decoupling tight and adherens junctions and opening the BBB transiently.
- Optical Methods: Laser interstitial thermotherapy and photodynamic therapy induce local heating, resulting in BBB modulation.
- Electrical Stimulation: Electroporation with electrodes can permeabilize BBB by generating short electrical pulses, increasing trans-endothelial transport.
- Mechanical Forces: Traumatic brain injury (TBI) represents a pathological physical disruption associated with shear and tensile forces, leading to acute and chronic BBB breakdown.
Key Features of Physical Disruption
- High selectivity and reversibility; permeability can be targeted to specific brain regions.
- Minimally invasive; for modalities like FUS, non-invasive targeting is possible.
- Duration and extent are adjustable with protocol parameters (frequency, intensity, exposure time).
- Potential for repeated and controlled BBB opening with reduced systemic effects.
Clinical Example: Focused Ultrasound
| Method | Mechanism | Targeting | Applications |
|---|---|---|---|
| Focused Ultrasound + Microbubbles | Mechanical cavitation, decoupling of junction proteins | Deep-brain, highly localized | Drug, antibody, and gene delivery; glioblastoma therapy |
Advantages and Limitations
- Advantages: Site-specific delivery, reversibility, lower risk of systemic adverse effects.
- Limitations: Potential tissue heating, cavitation injury, equipment and protocol complexity; safety and long-term efficacy require further validation.
Comparative Analysis: Chemical vs Physical BBB Disruption
| Aspect | Chemical Disruption | Physical Disruption |
|---|---|---|
| Mechanism | Biochemical modulation and osmotic stress; non-specific | Energy-based (acoustic, electrical, optical, mechanical); region-specific |
| Onset/Duration | Rapid onset, short-lived | Rapid or programmable; adjustable duration |
| Targeting | Systemic or vascular territory | Localized, deep-brain targeting possible |
| Safety | Risks include edema, neurotoxicity, infection | Risks include tissue heating, microvascular damage |
| Ease of Application | Simple, familiar, cost-effective | Requires advanced imaging and equipment; complex protocols |
| Clinical Status | Limited adoption due to reproducibility and safety* | Promising, under active research and clinical trials |
Clinical Applications and Current Research
Both chemical and physical BBB disruption strategies have shown promise in enhancing CNS drug delivery. Key applications include:
- Glioblastoma and Brain Tumor Treatment: Chemotherapeutics administered after BBB opening, both chemical (mannitol) and physical (FUS).
- Neurodegenerative Disorders: Delivery of antibodies (e.g., for Alzheimer’s), nanoparticles, and gene therapy vectors.
- Stroke and Traumatic Injury: Understanding BBB disruption is key to managing inflammation and secondary damage post-TBI.
Recent research focuses on the optimization of parameters, safety profiles, and development of combined approaches (e.g., FUS-mediated nanoparticle delivery) for enhanced therapeutic index.
Safety Concerns and Limitations
While BBB disruption strategies enable potent CNS drug delivery, they are not without risks:
- Unintended Entry: Disrupting the BBB may allow toxins and pathogens into the brain.
- Edema and Neuroinflammation: Rapid permeability increase risks swelling and inflammatory responses.
- Cytotoxicity: Repeated or uncontrolled disruption may damage neural tissue or vasculature.
- Variable Restitution: BBB recovery times and integrity depend on method, dose, and patient-specific factors.
Safety profiles require rigorous preclinical and clinical validation, with close monitoring for neurobehavioral, cognitive, and vascular complications.
Future Directions
The future of BBB disruption lies in highly selective, minimally invasive, and reversible methods, harnessing advances in nanotechnology, imaging, and biomaterials:
- Smart nanoparticles that cross BBB using endogenous transport mechanisms without disruption.
- Combinatorial approaches integrating chemical stimuli with physical targeting for synergy.
- Next-generation FUS protocols paired with real-time imaging and AI for precision control.
- Personalized medicine: Adapting BBB modulation to patient-specific vascular and genetic profiles.
Ethical considerations and long-term safety will remain central to clinical translation. The ultimate goal is effective CNS drug delivery with minimal adverse impact on brain function and structure.
Frequently Asked Questions (FAQs)
Q: What is the primary difference between chemical and physical BBB disruption?
Chemical disruption uses pharmacologic agents to induce barrier permeability through biochemical changes, while physical disruption applies energy-based modalities (ultrasound, electrical pulses, lasers) for targeted, non-pharmacologic BBB opening.
Q: Is BBB disruption reversible?
Both chemical and physical disruption methods are designed to induce temporary BBB openings; the barrier typically regains integrity within minutes to hours, depending on technique, dose, and tissue condition.
Q: Which method is safer—chemical or physical?
Safety profiles differ by modality and clinical context. Physical techniques often offer more localized and controlled disruption, potentially reducing systemic risks, but both approaches require careful optimization to avoid neural damage or infection.
Q: Are these BBB disruption methods currently used in humans?
Chemical disruption with mannitol has clinical precedent, especially in oncology. Physical methods, particularly focused ultrasound, are undergoing clinical trials and show promise for future widespread use.
Q: Can BBB disruption lead to long-term brain damage?
While designed for transient disruption, repeat or uncontrolled BBB opening can cause neuroinflammation, edema, or cognitive impairment. Long-term safety studies are critical in ongoing research.
References
Read full bio of medha deb












