Understanding Treatment-Induced Neuropathy of Diabetes: Causes, Symptoms, and Management
Explore the causes, symptoms, diagnosis, and management of treatment-induced neuropathy in diabetes for safer glycemic control.
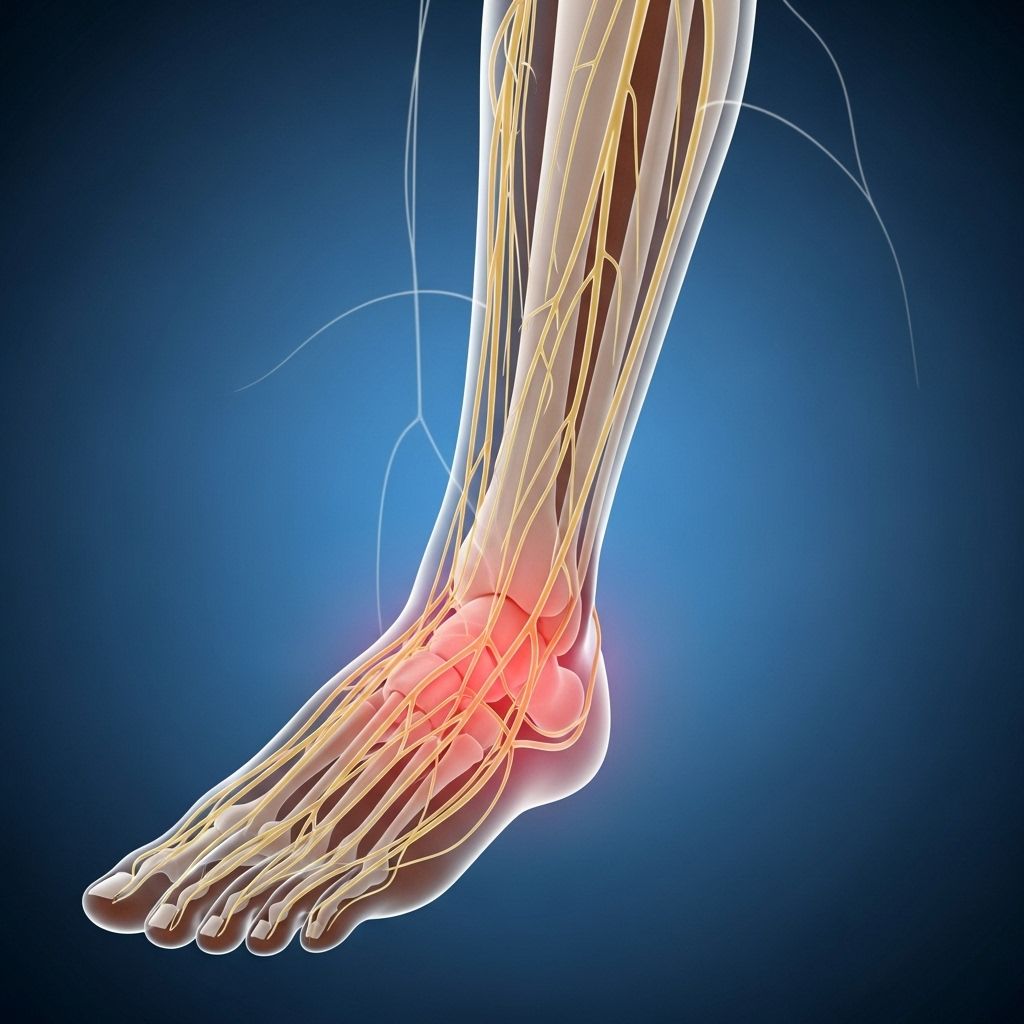
Treatment-Induced Neuropathy of Diabetes (TIND): An Overview
Treatment-induced neuropathy of diabetes (TIND), also known as insulin neuritis, is a rare but serious complication occurring after a rapid improvement in glycemic control in people with chronic hyperglycemia. Unlike other diabetic neuropathies, TIND typically develops acutely, often manifesting within eight weeks of a significant reduction in blood glucose levels. Awareness of TIND is crucial for both patients and healthcare providers, as it influences diabetes management decisions and patient quality of life.
What Is Treatment-Induced Neuropathy of Diabetes?
TIND is characterized by the acute onset of neuropathic pain and/or autonomic dysfunction following a notable decrease in blood sugar levels. The syndrome is usually triggered by aggressive attempts to lower HbA1c, the marker of average blood glucose over several months. For diagnosis, a rapid decrease in HbA1c of 2% points or more over three months in patients with previously uncontrolled diabetes is a hallmark, coinciding with new or worsening neurological symptoms.
Key Features of TIND
- Rapid onset: Symptoms appear typically within 8 weeks of glycemic improvement.
- Painful neuropathy: Sharp, burning pain, often asymmetrical, affecting distal limbs (feet and legs).
- Autonomic dysfunction: Symptoms such as orthostatic hypotension, abnormal sweating, gastrointestinal disturbances, or sexual dysfunction.
- Association with microvascular complications: Increased risk for retinopathy and microalbuminuria.
How Does TIND Differ from Other Types of Diabetic Neuropathy?
Diabetic neuropathy is a broad term encompassing several forms of nerve damage caused by prolonged high blood sugar. TIND is distinct due to its acute presentation and direct association with sudden improvement in glycemic control. Other forms, such as peripheral, autonomic, or proximal neuropathy, generally develop slowly with chronic uncontrolled diabetes.
| Neuropathy Type | Typical Onset | Cause | Main Symptoms |
|---|---|---|---|
| Treatment-Induced (TIND) | Acute (weeks) | Rapid glycemic improvement | Pain, autonomic dysfunction |
| Peripheral | Gradual (years) | Prolonged hyperglycemia | Numbness, tingling, ulcer risk |
| Autonomic | Gradual (years) | Prolonged hyperglycemia | GI, bladder, sexual issues |
Prevalence and Risk Factors
While TIND is underrecognized, studies estimate about 10% of people referred for diabetic neuropathy evaluation meet criteria for TIND. The principal risk factor is the magnitude of HbA1c reduction:
- 2–3% HbA1c decrease over 3 months: Approximately 20% risk of TIND
- >4% HbA1c decrease over 3 months: Risk exceeding 80%
- Duration of chronic hyperglycemia: Long-standing uncontrolled diabetes increases susceptibility to nerve damage with rapid correction.
- High baseline HbA1c: Greater disparity between initial and achieved glycemic levels heightens risk.
Underlying Causes and Mechanisms
The physical cause of TIND is believed to be related to compromised microvascular circulation that develops during chronic hyperglycemia. When glycemic control is rapidly restored, blood flow to nerves may change abruptly, resulting in ischemic injury and inflammation of small nerve fibers. This phenomenon is similar to other iatrogenic (treatment-caused) syndromes in medicine.
- Small fiber neuropathy: Damage mainly affects small sensory nerve fibers, producing intense pain and autonomic symptoms.
- Vascular response: Fast improvement in glucose can destabilize previously adapted blood vessels, leading to impaired nutrient and oxygen delivery to nerves.
Symptoms of Treatment-Induced Neuropathy of Diabetes
TIND can affect both sensory and autonomic nerves. The following are core symptoms:
- Neuropathic pain: Burning, stabbing, or electric-shock-like pain, commonly in the feet, legs, and sometimes hands.
- Allodynia & Hyperalgesia: Painful sensory response to normally non-painful stimuli (allodynia), or increased pain from mild stimuli (hyperalgesia).
- Autonomic dysfunction:
- Abnormal sweating or loss of ability to sweat (sudomotor dysfunction)
- Orthostatic hypotension (blood pressure drop when standing)
- Digestive disturbances (nausea, constipation, diarrhea)
- Genitourinary symptoms (erectile dysfunction, vaginal dryness)
- Microvascular complications: Rapid changes can worsen or precipitate diabetic retinopathy and microalbuminuria.
Severity and Course
The severity of pain and autonomic symptoms often correlates strongly with the degree of rapid HbA1c reduction. Higher decreases in HbA1c not only increase risk, but can lead to more pronounced symptoms, sometimes necessitating intensive pain management.
Diagnosing TIND
Diagnosis of TIND can be challenging due to its overlapping features with other types of diabetic neuropathy and the rarity of the condition. A combination of clinical history, laboratory findings, and specialized testing is frequently required.
- Clinical history: Recent rapid improvement in glycemic control with new onset pain or autonomic symptoms.
- Laboratory: Documentation of significant HbA1c drop (≥2% points within ~3 months).
- Neurological exam: Structured assessment of sensory and autonomic functions every 3–6 months.
- Small fiber function tests: Sudomotor testing (e.g., Sudoscan) may reveal small fiber damage in affected limbs.
- Exclusion: Exclude other causes of neuropathy such as vitamin deficiencies, toxin exposures, or concurrent illnesses.
Complications Associated with TIND
Aside from pain and dysautonomia, TIND is linked to a heightened risk for other diabetes complications after rapid glycemic change:
- Retinopathy: Sudden glycemic improvement can precipitate or worsen diabetic retinopathy.
- Microalbuminuria: Indicates early kidney involvement with microvascular complications.
- Functional impairment: Severe pain and orthostatic hypotension may reduce mobility and independence.
Prevention: How to Lower Risk of TIND
Currently, no formal clinical guidelines exist to prevent TIND. However, experts emphasize that glycemic control should be improved stepwise rather than abruptly, especially in individuals with chronic high HbA1c.
- Gradual reduction: Target a slower decline in blood sugar levels to allow nerves and microvasculature to adapt.
- Monitor symptoms: Regularly assess for neuropathic pain or autonomic symptoms during glycemic improvement.
- Collaborative management: Work with a diabetes care team for individualized adjustment of insulin or other therapies.
Treatment and Management of TIND
There are currently no established curative therapies for TIND. Management focuses on symptom relief, prevention of further complications, and supportive care.
Pain Management
- Medications:
- Pain relievers (paracetamol, NSAIDs)
- Neuropathic pain medications: pregabalin, duloxetine, gabapentin
- Case reports show improvement with increased pregabalin and addition of duloxetine.
- Alternative therapies:
- Acupuncture and physical therapy for supportive relief
- Psychological support for coping with chronic pain
Managing Autonomic and Other Complications
- Orthostatic hypotension: Adjust salt and fluid intake, use compression garments, and consider medications under medical guidance.
- Digestive issues: Dietary modifications—smaller, more frequent meals and limiting problematic foods.
- Genitourinary symptoms: Lubricants, medications, and specialist referral as needed.
Lifestyle and General Diabetes Care
- Foot care: Monitor feet for ulcers or injuries and seek prompt treatment for any wounds.
- Regular monitoring: Wellscheduled diabetes and neuropathy checkups to monitor complications.
- Quit smoking and exercise: Both help improve overall vascular health and slow progression of neuropathy.
- Collaborate with healthcare team: Adjust therapy regimens based on individualized risk and ongoing symptoms.
Frequently Asked Questions (FAQs)
What triggers treatment-induced neuropathy of diabetes?
TIND is typically triggered by a rapid decline in blood sugar, often after initiation or intensification of insulin therapy or other controlled diabetes treatments, especially in those with previously high HbA1c.
How is TIND different from traditional diabetic neuropathy?
Traditional diabetic neuropathy unfolds slowly due to long-term uncontrolled blood sugar. TIND, conversely, arises acutely following rapid glucose corrections.
Can TIND be prevented?
No established guidelines exist, but gradual and monitored reduction of blood sugar is currently the best practice for prevention.
Does everyone with rapid glycemic improvement develop TIND?
No. While risk increases with the magnitude of HbA1c reduction, not all patients experience the condition. Monitoring and individualized care can reduce incidence.
Can prediabetes cause neuropathy?
Some research suggests prediabetes can contribute to neuropathy, especially with prolonged high and uncontrolled blood sugar, but classic TIND is seen only after rapid glycemic correction in longstanding diabetes.
What should patients ask their doctors before making changes in diabetes treatment?
- — What are the risks of rapid sugar reduction in my case?
- — How can we monitor for neuropathy symptoms?
- — What steps can I take to minimize complications during glycemic improvement?
Key Takeaways
- TIND is an acute neuropathy caused by rapid improvement in blood glucose in people with uncontrolled diabetes.
- Symptoms include severe pain, autonomic dysfunction, and risk of worsening other diabetes complications.
- Prevention centers on gradual glycemic improvement and symptom monitoring.
- Treatment focuses on pain control, managing autonomic symptoms, and collaborative care.
References
- https://pubmed.ncbi.nlm.nih.gov/25392197/
- https://pubmed.ncbi.nlm.nih.gov/37967926/
- https://www.healthline.com/health/type-2-diabetes/diabetic-neuropathy
- https://www.everlywell.com/blog/hba1c/can-prediabetes-cause-neuropathy/
- https://www.painscale.com/article/conventional-treatment-options-for-peripheral-neuropathy
- https://www.healthline.com/health/video/diabetic-peripheral-neuropathy-symptoms
- https://www.medicalnewstoday.com/articles/245310
- https://www.healthline.com/health/video/top-things-to-know-about-diabetes-complications
- https://my.clevelandclinic.org/health/diseases/7104-diabetes
Read full bio of medha deb